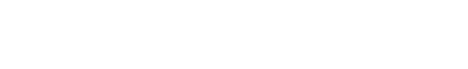Case Report
Authors: Nicholas W. DeKorver M.D., Ph.D.1, Michelle M. Lee, M.D.1, Grace M. Tabatabai, M.D.1, Tanner H. Hoke, M.D.1, Kimberly Mills, Pharm.D., BCPPS2, Tammara King BSN, RN1, Amelia Bray-Aschenbrenner, M.D.3, Ananth K. Vellimana, M.D.1,4,5, Kristin P. Guilliams, M.D., MSCI1, Maria M. Galardi, M.D.1
1 Department of Neurology, Division of Pediatric and Development Neurology, Washington University School of Medicine, Saint Louis MO, USA
2 Department of Pharmacy, St. Louis Children’s Hospital, St. Louis, MO, USA
3 Department of Pediatrics, Division of Emergency Medicine, Washington University School of Medicine, Saint Louis MO, USA
4 Department of Neurological Surgery, Washington University School of Medicine, St. Louis, MO.
5 Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, MO.
Corresponding author
Maria M. Galardi, M.D.
Mailing Address: 600 S. Euclid, Saint Louis, MO, 63110
Email Address: mmgalardi@wustl.edu
Telephone: 314-454-6120
Fax: 314-454-4225
Copyright © belongs to author(s)
All rights reserved.
Any redistribution or reproduction of part or all of the contents in any form is prohibited other than the following:
- you may print or download to a local hard disk extracts for your personal and non-commercial use only
- you may copy the content to individual third parties for their personal use, but only if you acknowledge the website as the source of the material
You may not, except with our express written permission, distribute or commercially exploit the content. Nor may you transmit it or store it in any other website or other form of electronic retrieval system.
Abstract
Pediatric acute ischemic stroke is a significant cause of morbidity and mortality in the pediatric population. The management of acute ischemic stroke in pediatric patients can include reperfusion therapies including use of tissue plasminogen activator and/or mechanical thrombectomy. Recently, many hospital systems have changed from tissue plasminogen activator to tenecteplase as the preferred fibrinolytic therapy. There is limited data on the use of tenecteplase in pediatric patients. Here, we report the safe use of tenecteplase followed by mechanical thrombectomy in a pediatric patient with acute ischemic stroke, as most prior reports have been limited to adult patients.
Introduction
Childhood stroke is a significant cause of morbidity and mortality in the pediatric population with estimates of prevalence ranging from 1.3 to 13 strokes per 100,0001–12. The etiologies for pediatric stroke differ greatly from adult counterparts. While there are pediatric populations at greater risk for stroke, including those with sickle cell disease, congenital and acquired heart disease, trauma, non-atherosclerotic arteriopathy, and infection, roughly one tenth to one third of childhood strokes are cryptogenic13–15. Like adult counterparts, children with stroke often present with focal deficits. However, up to two-thirds present with diffuse neurologic signs such as altered mental status or headache and roughly one-third present with seizures14,16,17. Additionally, children can present with a stuttering symptom course. Taken together, these factors complicate the timely recognition of stroke within a critical window to qualify for early reperfusion therapies including fibrinolytics and mechanical thrombectomy which can drastically improve outcomes.
While the management of adult stroke is based on large clinical trials, there is less evidence for the management of pediatric stroke. Recommendations are based on data from numerous smaller studies and extrapolation of adult stroke data. There are no large-scale clinical trials demonstrating the safety and efficacy of reperfusion therapies, including use of tissue plasminogen activator (tPA) and/or mechanical thrombectomy (MT) for treatment of pediatric stroke. However, in 2019, the American Heart Association/American Stroke Association provided a statement addressing controversies surrounding the use of hyperacute therapies in pediatric stroke, with clinical practice considerations including criteria for these interventions18. These criteria, in conjunction with expert consensus based on analysis of retrospective data, have allowed for the development of institutional protocols to treat pediatric stroke19–26. Importantly, from a safety perspective, treatment with tPA within 4.5 hours of stroke onset is associated with a low risk of symptomatic intracranial hemorrhage27.
Tenecteplase (TNK) is becoming the thrombolytic of choice in adult stroke centers, but pediatric data on its use is currently very limited. Tenecteplase is a genetically modified form of tissue plasminogen activator protein with more favorable properties including higher fibrin specificity, longer half-life allowing for single bolus dosing, and improved resistance to plasminogen activator inhibitor-128–30. Several large clinical trials and meta-analyses have demonstrated that TNK is safe, effective, and possibly superior to tPA for treatment of adult acute stoke31–35. Based on a review of 26 studies, the risk of intracranial hemorrhage with TNK treatment in adult stroke varies based on dose ranging from 0.99% to 4.19%, which is comparable to risk with tPA treatment36. There is no data within the pediatric stroke population for dosing, safety, or efficacy of TNK use. For TNK use in pediatric populations, the limited data that exists is drawn from case series demonstrating apparent safety of TNK infusion for intravascular thrombus (venous thromboembolism and arterial thrombosis) and as a bolus for treatment of intra-operative procedural stent thrombosis37,38.
While the transition from the use of tPA to TNK for the treatment of adult acute ischemic stroke (AIS) has been rapidly studied and supported with increasing evidence, to our knowledge, there are no documented reports or on-going trials on the safety and efficacy of treatment of pediatric AIS with TNK. Despite growth and increased coordination amongst the pediatric stroke field, the early use of TNK in pediatric stroke still relies on expert opinion, review of retrospective case studies, and institutional protocols. Here, we present a clinical case detailing the safe delivery of TNK followed by mechanical thrombectomy for treatment of AIS in a pediatric patient.
Case Report
A 13-year-old female with a past medical history of depression presented to the pediatric emergency department with acute onset left-sided weakness involving the face, arm, and leg. She had been in her normal state of health prior to the onset of weakness causing the patient to collapse while doing push-ups. Her collapse was witnessed by a sibling who immediately alerted the family resulting in prompt presentation and emergent evaluation.
On arrival to the emergency room, the patient was noted to have right gaze deviation and left-sided weakness involving the face, arm, and leg prompting code stroke activation for expedited evaluation by neurology. Initial vital signs were normal. Blood glucose was 113. Her last known normal was roughly 60 minutes prior to presentation. She had an initial NIH Stroke Scale (NIHSS) score of 13 at 60 minutes from last known well (LKW) (Table 1). Non-contrast head CT was negative for hemorrhage and had no early evidence of ischemic change (Figure 1a). Hyperacute MRI with time-of-flight MRA revealed a right M1 occlusion with diffusion restriction within the right lentiform nucleus and right inferior frontal and insular cortices without FLAIR changes, consistent with a hyperacute stroke secondary to large vessel occlusion of the right middle cerebral artery (MCA) (Figures 1b and 1c). Repeat NIHSS at 1.5 hours from LKW and following imaging marginally increased to 16 with progression to no anti-gravity movement of the left upper extremity and new drift of right lower extremity (Table 1). Labs including complete blood count (CBC), basic metabolic panel (BMP), and coagulation studies were normal. Her urine HCG and drug screen were negative. As the patient was within therapeutic window for treatment with TNK and mechanical thrombectomy for management of the proximal large vessel occlusion (LVO), her case was reviewed urgently by two pediatric stroke attendings. The risks and benefits were discussed with the family and there was agreement that the potential benefits of thrombolytic therapy outweighed the risks. The patient was treated with intravenous (IV) TNK (0.25 mg/kg) with a door-to-needle time of 90 minutes (time from stroke onset of 2.5 hours).
Following the administration of TNK and discussion with interventional neuroradiology, the patient was taken for percutaneous arterial mechanical thrombectomy around 3 hours from stroke onset. Initial angiography demonstrated occlusion of the proximal right MCA M1 segment (Figure 1d). Following pass 1, the patient had reperfusion of the M1 and inferior M2 division with a persistent superior division M2 occlusion along with an interval right A1 segment occlusion. She underwent 5 passes with the final pass revealing reperfusion of the right MCA territory with persistent occlusion of a superior M2 division and posterior M3 division (Figure 1d). Reperfusion was classified as mTICI2b (modified Thrombolysis in Cerebral Infarction score 2b) given reperfusion of greater than half the vascular distribution of the occluded artery with some incomplete filling distally39. The patient was admitted to the pediatric intensive care unit for post-thrombectomy monitoring.
Her follow-up NIHSS at roughly 24 hours from stroke onset was 11 (Table 1). Post-intervention non-contrasted head CT at 12 and 36 hours revealed evolution of ischemic injury without intracranial hemorrhage. She was started on aspirin 81 mg daily 24 hours after TNK administration. NIHSS remained stable with a score of 11 at roughly 2 days from LKW (Table 1). She underwent an extensive evaluation in efforts to determine acute stroke etiology. Inflammatory and autoimmune markers including erythrocyte sedimentation rate and c-reactive protein, anti-nuclear antigen, extractable nuclear antigen, antineutrophilic cytoplasmic antibody, double-stranded DNA, complement 3 and complement 4 were normal. Respiratory viral panel was negative. A thorough hypercoagulability evaluation including anti-phospholipid panel (lupus anti-coagulant, anti-cardiolipin, beta-2-glycoprotein antibodies), protein c resistance, hemoglobin electrophoresis, protein C, protein S, anti-thrombin III level, factor VIII level, and homocysteine was unrevealing. Renal and bilateral upper and lower extremity venous doppler imaging were also normal. A cardiac evaluation including transthoracic and transesophageal echocardiograms with bubble study to evaluate for intracardiac and intrapulmonary shunting was normal. Evaluation for traumatic etiologies included a CTA head and neck did not demonstrate any evidence of dissection. As shown in Figure 2, repeat brain MRI and MRA with dedicated vessel wall imaging 3 days post-infarction demonstrated an evolving right MCA territory infarct with cytotoxic edema and mild mass effect, as well as long segment circumferential enhancement along the right internal carotid and irregularity and abnormal flow in the right MCA. (Figure 2a,b,c). Given there was no pre-thrombectomy contrasted imaging and initial MRA showed a proximal M1 LVO, it was unclear if vessel wall irregularity and enhancement pre-dated the thrombectomy and was indicative of focal cerebral arteriopathy (FCA), or if the enhancement was secondary to irritation of the vessel wall during the procedure. After multidisciplinary discussion, it was felt that risks of not treating for possible FCA outweighed the risk of treatment. The patient received 3 days of high-dose IV steroids followed by a 4-week steroid taper without complications. The patient was evaluated by genetics and rapid whole exome testing was negative. Overall, the etiology of our patient’s stroke remains undetermined.
Following clinical stabilization, the patient was admitted to an inpatient pediatric neuro-rehabilitation program for intensive therapies. She was started on gabapentin for neuropathic pain and fluoxetine for exacerbation of premorbid anxiety and depression. Repeat brain MRI 1 month post-stroke demonstrated an evolving right MCA territory infarct with areas suspicious for hemorrhagic conversion, interval decreased mass effect and midline shift, mild irregular narrowing of the right distal M1 and M2 segments with decreased flow in the right M3/M4 segments, arterial wall enhancement within the visualized right internal carotid artery, and subtle asymmetric enhancement of the right MCA wall (Figure 3a,b,c). The patient made significant motor recovery with intensive multi-disciplinary therapies. Her Modified Rankin Scale (MRS), a measure of neurologic disability, at the time of discharge was 3 with a NIHSS of 5 (Table 1). Her pediatric stroke outcome measure (PSOM) score at the time of discharge was 1.5 for sensory motor (hemiparesis, hemifacial, dysarthria, hemisensory) and behavioral (inattention) deficits. Repeat brain MRI 3-month post-stroke showed decreased arterial wall enhancement involving right ICA and M1/M2 segments, as well as improved flow in right M3/M4 segments.
Discussion
To our knowledge, this is one of the first case reports of TNK administration for the treatment of pediatric acute stroke. Our patient was treated with 0.25 mg/kg TNK as a single bolus at roughly 2.5 hours post-symptom onset. She tolerated the bolus without complications and was taken for mechanical thrombectomy resulting in mTICI2b reperfusion. There was no evidence of hemorrhagic conversion on the 12-hour or 36-hour post-intervention CT. This case serves as a demonstration of the safe use of TNK followed by thrombectomy for AIS in a pediatric patient.
Clinical trials in the adult stroke population have shown that the change from tPA to TNK for thrombolytic treatment of AIS is safe and effective, supporting its increased use as the fibrinolytic of choice at stroke centers. A recent meta-analysis looking at over 3700 adult patients across nine different clinical trials concluded that TNK has similar safety and efficacy to tPA40. A 2023 study of over 9000 adults showed decreased risk of symptomatic intracranial hemorrhage for those who received TNK compared to tPA (1.8% versus 3.6%, respectively) with similar results in patients that received thrombectomy and those who did not41. While this is compelling evidence supporting TNK use in adult populations, there is no such data demonstrating safe use in pediatric stroke. There are several important factors complicating the extrapolation of data pertaining to the use of fibrinolytics from adult stroke patients to pediatric patients, including differences in coagulation and fibrinolysis pathways, age-dependent differences in plasminogen, plasminogen activator inhibitor-1, and tPA levels/activity, differing volume of distribution, and potential differences in hepatic clearance42,43. The Thrombolysis in Pediatric Stroke (TIPS) trial was designed to investigate the potential impact of these developmental differences with a range of tPA doses, but unfortunately was stopped before data collection was completed44. However, per the Thrombolysis in Pediatric Stroke Extended Registry (TIPSTER), when adult-equivalent selection criteria are applied to children, the risk of symptomatic intracranial hemorrhage seems to be low27. It is reasonable to expect similar rates with TNK given the equivalency of safety data between tPA and TNK in adults, but this will need to be verified with prospective, pediatric-specific data.
Expert consensus and provider experience are important, albeit variable, factors in the development of institutional treatment algorithms for pediatric stroke. In more recent efforts to characterize the pediatric stroke expert experience regarding the use of thrombolytics for pediatric stroke, the International Pediatric Stroke Organization (IPSO) published a survey of its members that showed while 72% of respondents reported willingness to treat children with acute stroke with TNK, only two respondents had experience caring for pediatric patients who received TNK. One was a 9-year-old who was initially treated at an adult stroke center prior to transfer to a pediatric facility, but later identified to have a stroke mimic; the other a 14-year-old with no additional clinical information reported45.
We also recognize a challenge in wide implementation of thrombolytic treatment algorithms depending on the availability of advanced neuroimaging. Our patient benefitted from rapid evaluation and treatment decision because she presented to a tertiary pediatric hospital with expertise and infrastructure consistent with recommended components of a comprehensive pediatric stroke program46, including a hyperacute MRI protocol developed for identification of acute stroke and large vessel occlusions. At many centers, this advanced imaging may not be available and treatment decisions must be made based on other imaging modalities such as CT. Adult data on TNK use demonstrates similar or improved risk of ICH compared to tPA, which is often given for treatment of acute stroke in adults without confirmation of stroke on advanced imaging. In situations where advanced imaging is not available or plausible within therapeutic time window, decisions should be made on a case-by case basis within each institution’s framework, taking into account clinical suspicion for stroke versus stroke mimic and patient’s individual characteristics, including exclusion of intracranial hemorrhage, which can be accomplished with head CT.
The timing of contrasted imaging acquisition relative to interventions complicates the definitive etiology for her stroke. As our patient did not receive contrasted imaging prior to MT, it remains unclear if enhancement and irregularity of the vessel wall noted on post-thrombectomy contrasted studies was a treatment effect from multi-pass thrombectomy or related to an underlying process such as FCA. The safety and utility of thrombectomy or thrombolysis in AIS secondary to LVO related to FCA remains unclear. One study demonstrated low thrombectomy procedural hemorrhagic risk comparable to patients with cardioembolic stroke, but with a higher rate of re-occlusion or persistent >50% stenosis on follow-up imaging suggesting thrombectomy may be less effective in patients with FCA47. In our patient, FCA could be a potential mechanism for why despite early and aggressive interventions, she still had a large stroke with persistent functional deficits.
The clear limitation to this report is that it is based on the treatment of a single patient at one institution. While large, randomized prospective studies demonstrating the safe and effective use of TNK with or without subsequent thrombectomy are ideal to assess the overall safety and efficacy, these studies are unlikely due to the relatively low incidence of pediatric stroke. However, as pediatric stroke networks grow and evaluation pathways for timely treatment improve, future multi-center cohort studies may allow for prospective studies on standardized protocols and the use of TNK for pediatric stroke.
Conclusion
This case demonstrates the safe use of TNK followed by thrombectomy in the treatment of pediatric AIS. Our patient had noted improvement in function following treatment with TNK, thrombectomy, and inpatient neurorehabilitation, but did have residual motor deficits, suggesting unclear efficacy of TNK for this particular patient. Prospective studies are needed to determine the safety and efficacy of TNK in pediatric stroke.
Acknowledgements:
Author Contributions: Draft preparation and review was completed by NWD, MML, GMT, THH, AA, AKV, KPG, and MMG. All authors discussed reviewed and contributed to the final manuscript.
Conflicts of interest Statement: No conflict of interest for NWD, MML, GMT, THH, AA, AKV, KPG, and MMG.
References
- Lehman LL, Khoury JC, Taylor JM, et al. Pediatric Stroke Rates Over 17 Years: Report From a Population-Based Study. J Child Neurol. 2018;33(7):463-467. doi:10.1177/0883073818767039
- Giroud M, Lemesle M, Gouyon JB, Nivelon JL, Milan C, Dumas R. Cerebrovascular disease in children under 16 years of age in the city of Dijon, France: a study of incidence and clinical features from 1985 to 1993. J Clin Epidemiol. 1995;48(11):1343-1348. doi:10.1016/0895-4356(95)00039-9
- Eeg-Olofsson O, Ringheim Y. Stroke in children. Clinical characteristics and prognosis. Acta Paediatr Scand. 1983;72(3):391-395. doi:10.1111/j.1651-2227.1983.tb09734.x
- Broderick J, Talbot GT, Prenger E, Leach A, Brott T. Stroke in children within a major metropolitan area: the surprising importance of intracerebral hemorrhage. J Child Neurol. 1993;8(3):250-255. doi:10.1177/088307389300800308
- Zahuranec DB, Brown DL, Lisabeth LD, Morgenstern LB. Is it time for a large, collaborative study of pediatric stroke? Stroke. 2005;36(9):1825-1829. doi:10.1161/01.STR.0000177882.08802.3c
- Steinlin M, Pfister I, Pavlovic J, et al. The first three years of the Swiss Neuropaediatric Stroke Registry (SNPSR): a population-based study of incidence, symptoms and risk factors. Neuropediatrics. 2005;36(2):90-97. doi:10.1055/s-2005-837658
- Chung B, Wong V. Pediatric stroke among Hong Kong Chinese subjects. Pediatrics. 2004;114(2):e206-212. doi:10.1542/peds.114.2.e206
- Grunt S, Mazenauer L, Buerki SE, et al. Incidence and outcomes of symptomatic neonatal arterial ischemic stroke. Pediatrics. 2015;135(5):e1220-1228. doi:10.1542/peds.2014-1520
- Earley CJ, Kittner SJ, Feeser BR, et al. Stroke in children and sickle-cell disease: Baltimore-Washington Cooperative Young Stroke Study. Neurology. 1998;51(1):169-176. doi:10.1212/wnl.51.1.169
- Agrawal N, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke. 2009;40(11):3415-3421. doi:10.1161/STROKEAHA.109.564633
- Barnes C, Newall F, Furmedge J, Mackay M, Monagle P. Arterial ischaemic stroke in children. J Paediatr Child Health. 2004;40(7):384-387. doi:10.1111/j.1440-1754.2004.00407.x
- Sporns PB, Fullerton HJ, Lee S, et al. Childhood stroke. Nat Rev Dis Primer. 2022;8(1):12. doi:10.1038/s41572-022-00337-x
- Sun LR, Jordan LC. Cryptogenic Pediatric Ischemic Stroke: What’s the Hole Story? Neurology. 2021;97(21):973-974. doi:10.1212/WNL.0000000000012960
- Mackay MT, Wiznitzer M, Benedict SL, et al. Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Ann Neurol. 2011;69(1):130-140. doi:10.1002/ana.22224
- Williams LS, Garg BP, Cohen M, Fleck JD, Biller J. Subtypes of ischemic stroke in children and young adults. Neurology. 1997;49(6):1541-1545. doi:10.1212/wnl.49.6.1541
- Fox C. Pediatric Ischemic Stroke. Contin Minneap Minn. 2023;29(2):566-583. doi:10.1212/CON.0000000000001239
- Yock-Corrales A, Babl FE, Mosley IT, Mackay MT. Can the FAST and ROSIER adult stroke recognition tools be applied to confirmed childhood arterial ischemic stroke? BMC Pediatr. 2011;11:93. doi:10.1186/1471-2431-11-93
- Ferriero DM, Fullerton HJ, Bernard TJ, et al. Management of Stroke in Neonates and Children: A Scientific Statement From the American Heart Association/American Stroke Association. Stroke. 2019;50(3):e51-e96. doi:10.1161/STR.0000000000000183
- Barry M, Barry D, Kansagra AP, et al. Higher-Quality Data Collection Is Critical to Establish the Safety and Efficacy of Pediatric Mechanical Thrombectomy. Stroke. 2021;52(4):1213-1221. doi:10.1161/STROKEAHA.120.032009
- Bhatia K, Kortman H, Blair C, et al. Mechanical thrombectomy in pediatric stroke: systematic review, individual patient data meta-analysis, and case series. J Neurosurg Pediatr. Published online August 9, 2019:1-14. doi:10.3171/2019.5.PEDS19126
- Surtees TL, Pearson R, Harrar DB, Lee S, Amlie-Lefond CM, Guilliams KP. Acute Hospital Management of Pediatric Stroke. Semin Pediatr Neurol. 2022;43:100990. doi:10.1016/j.spen.2022.100990
- Janjua N, Nasar A, Lynch JK, Qureshi AI. Thrombolysis for ischemic stroke in children: data from the nationwide inpatient sample. Stroke. 2007;38(6):1850-1854. doi:10.1161/STROKEAHA.106.473983
- Amlie-Lefond C, deVeber G, Chan AK, et al. Use of alteplase in childhood arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol. 2009;8(6):530-536. doi:10.1016/S1474-4422(09)70106-1
- Amlie-Lefond C, Wainwright MS. Organizing for Acute Arterial Ischemic Stroke in Children. Stroke. 2019;50(12):3662-3668. doi:10.1161/STROKEAHA.119.025497
- Amlie-Lefond C, Wainwright MS. Response by Amlie-Lefond and Wainwright to Letter Regarding Article, “Organizing for Acute Arterial Ischemic Stroke in Children.” Stroke. 2020;51(2):e37. doi:10.1161/STROKEAHA.119.028380
- Sporns PB, Wildgruber M, Kemmling A. Letter by Sporns et al Regarding Article, “Organizing for Acute Arterial Ischemic Stroke in Children.” Stroke. 2020;51(2):e36. doi:10.1161/STROKEAHA.119.028320
- Amlie-Lefond C, Shaw DWW, Cooper A, et al. Risk of Intracranial Hemorrhage Following Intravenous tPA (Tissue-Type Plasminogen Activator) for Acute Stroke Is Low in Children. Stroke. 2020;51(2):542-548. doi:10.1161/STROKEAHA.119.027225
- Li S, Pan Y, Wang Z, et al. Safety and efficacy of tenecteplase versus alteplase in patients with acute ischaemic stroke (TRACE): a multicentre, randomised, open label, blinded-endpoint (PROBE) controlled phase II study. Stroke Vasc Neurol. 2022;7(1):47-53. doi:10.1136/svn-2021-000978
- Logallo N, Kvistad CE, Thomassen L. Therapeutic Potential of Tenecteplase in the Management of Acute Ischemic Stroke. CNS Drugs. 2015;29(10):811-818. doi:10.1007/s40263-015-0280-9
- Llevadot J, Giugliano RP, Antman EM. Bolus fibrinolytic therapy in acute myocardial infarction. JAMA. 2001;286(4):442-449. doi:10.1001/jama.286.4.442
- Sun LR, Wilson JL, Waak M, et al. Tenecteplase in Acute Stroke: What About the Children? Stroke. 2023;54(7):1950-1953. doi:10.1161/STROKEAHA.123.042951
- Warach SJ, Dula AN, Milling TJ, et al. Prospective Observational Cohort Study of Tenecteplase Versus Alteplase in Routine Clinical Practice. Stroke. 2022;53(12):3583-3593. doi:10.1161/STROKEAHA.122.038950
- Yogendrakumar V, Churilov L, Guha P, et al. Tenecteplase Treatment and Thrombus Characteristics Associated With Early Reperfusion: An EXTEND-IA TNK Trials Analysis. Stroke. 2023;54(3):706-714. doi:10.1161/STROKEAHA.122.041061
- Ma P, Zhang Y, Chang L, et al. Tenecteplase vs. alteplase for the treatment of patients with acute ischemic stroke: a systematic review and meta-analysis. J Neurol. 2022;269(10):5262-5271. doi:10.1007/s00415-022-11242-4
- Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet Lond Engl. 2022;400(10347):161-169. doi:10.1016/S0140-6736(22)01054-6
- Rose D, Cavalier A, Kam W, et al. Complications of Intravenous Tenecteplase Versus Alteplase for the Treatment of Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Stroke. 2023;54(5):1192-1204. doi:10.1161/STROKEAHA.122.042335
- Tan JWY, Alwi M, Siew ELL, Samion H. Role of tenecteplase (rtPA) to re-establish flow in intraprocedural stent thrombosis in infants undergoing ductal stenting for duct-dependent pulmonary circulation-a case series. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2021;98(4):738-742. doi:10.1002/ccd.29838
- Hooper E, Noonan P, Caylor K, Gharpure V. 1270: Tenecteplase Use in Children with Intravascular Thrombosis. Crit Care Med. 2013;41:A326-A327. doi:10.1097/01.ccm.0000440502.06185.94
- Almekhlafi MA, Mishra S, Desai JA, et al. Not all “successful” angiographic reperfusion patients are an equal validation of a modified TICI scoring system. Interv Neuroradiol J Peritherapeutic Neuroradiol Surg Proced Relat Neurosci. 2014;20(1):21-27. doi:10.15274/INR-2014-10004
- Kobeissi H, Ghozy S, Turfe B, et al. Tenecteplase vs. alteplase for treatment of acute ischemic stroke: A systematic review and meta-analysis of randomized trials. Front Neurol. 2023;14:1102463. doi:10.3389/fneur.2023.1102463
- Warach SJ, Ranta A, Kim J, et al. Symptomatic Intracranial Hemorrhage With Tenecteplase vs Alteplase in Patients With Acute Ischemic Stroke: The Comparative Effectiveness of Routine Tenecteplase vs Alteplase in Acute Ischemic Stroke (CERTAIN) Collaboration. JAMA Neurol. 2023;80(7):732-738. doi:10.1001/jamaneurol.2023.1449
- Dodds WJ, Moynihan AC, Benson RE, Hall CA. The value of age- and sex-matched controls for coagulation studies. Br J Haematol. 1975;29(2):305-317. doi:10.1111/j.1365-2141.1975.tb01825.x
- Siegbahn A, Ruusuvaara L. Age dependence of blood fibrinolytic components and the effects of low-dose oral contraceptives on coagulation and fibrinolysis in teenagers. Thromb Haemost. 1988;60(3):361-364.
- Rivkin MJ, deVeber G, Ichord RN, et al. Thrombolysis in pediatric stroke study. Stroke. 2015;46(3):880-885. doi:10.1161/STROKEAHA.114.008210
- Wilson, Jenny L. and Waak, Michaela and Barry, Megan and Jordan, Lori C. and Sun, Lisa R. Tenecteplase in Pediatric Stroke: Ready or Not. https://ssrn.com/abstract=4558937
- Roach ES, Bernard T, deVeber G. Defining a Pediatric Stroke Center. Pediatr Neurol. 2020;112:11-13. doi:10.1016/j.pediatrneurol.2020.08.008
- Kossorotoff M, Kerleroux B, Boulouis G, et al. Recanalization Treatments for Pediatric Acute Ischemic Stroke in France. JAMA Netw Open. 2022;5(9):e2231343. doi:10.1001/jamanetworkopen.2022.31343
Table/Figure Legends
Table 1. Pediatric National Institutes of Health Pediatric Stroke Scale (PedNIHSS) scores at presentation and select points following acute treatment with TNK and Thrombectomy. Times are designated as time from last known well (LKW). Patient had presenting stroke score of 13 at 60 minutes from LKW, most notable for altered mental status, left sided facial droop, and left sided extremity weakness congruent with right MCA territory infarction. She had mild worsening over the next hour while undergoing initial stroke evaluation. PedNIHSS was slightly improved at 24 hours and remained stable at 2 days. Following inpatient rehabilitation, the patient had significant improvement and was discharged with PedNIHSS of 5 for mild left facial droop, left upper extremity weakness, and mild sensory deficit.
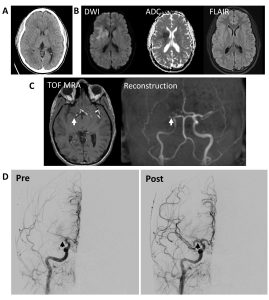
Figure 1: Neuroimaging and conventional angiography for 13-year-old girl with acute onset left sided weakness with suspicious for acute stroke. A. Initial non-contrast full dose head CT was negative for hemorrhage or acute pathology. B. Brain MRI without contrast showing diffusion restriction in the right lentiform nucleus and right inferior frontal and insular cortices without FLAIR changes consistent with hyperacute stroke. C. Time of flight MRA showing right MCA cutoff (arrows). C. Conventional angiography with pre-pass imaging on the left showing lack of distal flow consistent with occlusion of the right M1 MCA segment and post-thrombectomy showing TICI2b reperfusion in right MCA distribution (arrows). There was persistent occlusion of superior right M2 division and posterior M3 division.
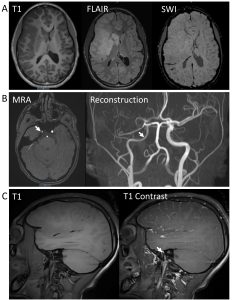
Figure 2: Follow up neuroimaging at 3 days post-thrombectomy. A. Brain MRI showing large right MCA territory infarction with swelling and mass effect with developing hypo intensity on T1 imaging, hyperintensity throughout MCA distribution on FLAIR, and no definitive hemorrhage conversion on susceptibility weight imaging (SWI). B. MRA source imaging and reconstruction showing reperfusion of the proximal segments of the right MCA, no flow in right M3 or M4 segments and asymmetric distal ICA (arrows). C. Long segment circumferential wall thickening with enhancement along the right internal carotid artery (arrow).
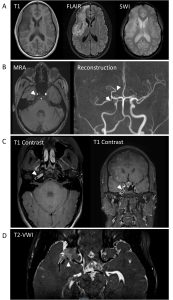
Figure 3: Follow up neuroimaging at 1-month post-thrombectomy. A. Brain MRI demonstrating evolving right MCA territory infarction with decreased mass effected seen as hypo intensity on T1 imaging and hyperintensity on FLAIR with areas suspicious for small hemorrhage within the area of infection on susceptibility weighted imaging (SWI) (white arrow). B. MRA source image and reconstruction demonstrating mild irregular narrowing of the right MCA M1 and M2 segments (white arrows) with decreased flow in the right M3/M4 segments. C. T1 contrasted images showing arterial wall enhancement within the visualized right internal carotid artery (white arrows). D. Vessel wall enhancement (white arrow) demonstrated on T2 based vessel wall imaging (VWI).
Administration of Tenecteplase Followed by Mechanical Thrombectomy