Original Research
Lori Xu, MD, Pediatric Neurology Resident, University of Texas Southwestern Medical Center, Dallas, Texas, USA
Wilmot, Bonnet, MD. Assistant Professor of Pediatrics and Neurology, University of Texas Southwestern Medical Center, Dallas, Texas, USA
Michael M Dowling, MD, PhD, Professor of Pediatrics and Neurology, University of Texas Southwestern Medical Center, Dallas, Texas, USA
Corresponding author
Michael M Dowling, MD, PhD
Division of Pediatric Neurology
University of Texas Southwestern Medical Center
5323 Harry Hines Blvd.
Dallas, TX 75390-9063
Michael.dowling@utsouthwestern.edu
Abstract
Introduction: Moyamoya (MM) is a rare progressive stenosis of anterior cerebral circulation of unknown etiology that occurs at higher prevalence in patients with sickle cell disease, trisomy 21, neurofibromatosis 1, and East Asians.
Hypothesis: Our objective was to compare the prevalence of potential shunting in patients with MM to controls without MM. We hypothesized that vasoactive substances, normally inactivated in the lungs, can escape inactivation through potential right-to-left shunting and thereby act upon the endothelium in at-risk patients and contribute to the development of moyamoya. We would then predict that there would be higher prevalence of potential right-to-left shunting observed in our patients with MM compared to children without MM.
Methods: In an IRB-approved, retrospective case-control study we identified 70 MM patients who had echocardiograms between 1991-2022 and compared the prevalence of potential right-to-left shunting with that of a control group of 123 patients without MM or stroke from a prior study.
Results: In the MM group, 35/70 (50%) had potential shunting identified on echocardiogram (26 intracardiac and 9 intrapulmonary) versus 29/123 (24%) potential shunting in our control group (P=0.0001). Significantly higher prevalences of potential shunting remained in subgroup analyses for potential intracardiac (37.1% vs 18.7%, p=0.004) and potential intrapulmonary shunting (28.1% vs 5.7%. p=0.0002).
Conclusions: These observations support our hypothesis that shunting could contribute to moyamoya development, possibly through unknown vasoactive substances escaping inactivation in the pulmonary vascular bed, subsequently inducing vascular changes in patients with predisposing conditions. This could represent a potential “second hit” mechanism contributing to the development of moyamoya in predisposed patients. This has potential mechanistic and therapeutic implications and will need to be verified in larger prospective studies.
Introduction
Moyamoya (MM) is a rare chronic progressive stenosis that affects the large anterior cerebrovascular arteries of the Circle of Willis, most commonly the terminal bilateral internal carotid arteries, with subsequent development of compensatory prominent small collateral vessels. These small collateral vessels characteristically appear as a “puff of smoke” or “moyamoya” in Japanese on angiographic imaging. These progressive vessel changes increase the risk for stroke.1
Moyamoya was first described in Japan in the 1950s with geographical differences in incidence and prevalence. The highest prevalence is in Japan, China, and Korea, with an annual incidence up to 1 per 100,000 population, and prevalence approximately 10 per 100,000 population.1,2 The incidence in the United States was 0.57 per 100,000 persons per year,1–3 though the incidence for Asian Americans in the United States per one study was similar to that of Japan, whereas the rates were lower for Caucasians, African Americans, and Hispanics in the United States. Current studies suggest a bimodal distribution of age of onset for moyamoya, first around 10 years of age and again at 40 years of age.1 Both children and adults predominantly present with ischemic strokes, though adults are more likely to present with hemorrhagic stroke compared to children.1–3
MM disease (MMD) differs from MM syndrome (MMS). MMD is more prevalent in East Asians,4 where the disease is associated with mutations in the ring finger protein 213 (RNF213) gene and appears to be inherited in an autosomal dominant pattern with low penetrance.5 This low penetrance suggests that in individuals carrying the mutation in RNF213, other factors (a “second hit”) contribute to the development of the vasculopathy. This gene is not associated with Caucasian idiopathic MMS or other syndromic MM. The at-risk conditions known for increased prevalence syndromic moyamoya (MMS) include sickle cell disease (SCD, 13,000 – 30,000 per 100,000 population), Trisomy 21 (T21, 3,800 per 100,000 population), Neurofibromatosis 1 (NF1, 600 per 100,000 population), and patients with congenital heart anomalies.6–11 Studies suggest inflammatory, infectious, or genetic etiologies for both MMD and MMS, but there currently are no clear unifying factors associating MMD and MMS.1,3
It also remains unknown what risk factors connect the vasculopathy in these patients with these higher risk medical conditions. Inflammatory or vasoactive factors may be contributory in patients at risk due to their underlying medical conditions or genetic factors such as mutations in the RNF213 gene.1,12 We noted that the pulmonary vascular bed has significant enzymatic function and serves to inactivate a host of inflammatory and vasoactive factors.13 In the event of right-to-left shunting, [via patent foramen ovale (PFO), atrial septal defect (ASD), ventricular septal defects (VSD), or pulmonary arteriovenous malformations (pulmAVM)] such factors can enter the arterial circulation without inactivation and thus affect the brain and cerebral arteries directly. We hypothesized that the development of moyamoya in susceptible populations could be due to unknown substances (such as these inflammatory or vasoactive substances) that escape inactivation in the pulmonary vasculature via potential right-to-left shunting. With this escape/shunting, these factors can subsequently induce cerebral arterial changes in these susceptible populations, including those with RNF213 gene mutations, SCD, T21, NF1, or other susceptible conditions. If this shunting were a contributing factor, we would predict a higher prevalence of potential right-to-left shunting (including intracardiac and intrapulmonary shunting) in patients with moyamoya compared to controls without moyamoya.
Methods
To investigate this hypothesis, we performed a retrospective chart review of electronic medical records of patients with moyamoya seen between 1991 and 2022 at Children’s Health, Dallas, Texas to identify children <19 years old with a diagnosis of MMD or MMS who had undergone echocardiogram by any technique. Using this data, we performed a case-control study comparing the prevalence of potential right-to-left shunting in patient cases with MM versus that in controls without MM or stroke from a prior multicenter study.14
Both studies were approved by the Institutional Review Board of the University of Texas Southwestern, and informed consent provided or waived as appropriate.
Cases
Of our patients (n = 100) with moyamoya, we excluded patients who did not have clinically obtained echocardiogram or MM diagnosis prior to 19 years of age. Our remaining cases (n = 70) included patients with MM and echocardiograms. Not all cases had Doppler or contrasted echocardiograms. We reviewed only their clinically obtained studies.
Controls
Our control group consisted of 123 patients without stroke from a prior prospective multicenter observational study to detect potential right-to-left shunting in children. This is the only reported study of the echocardiographically-detectable prevalence of PFO, and other potential shunts in children without stroke.14 Inclusion criteria for these controls included patients who were “healthy” at the time of study but who did require the presence of an IV for another clinical indication for the normal saline contrasted transthoracic echocardiogram (“bubble study”).14 Exclusion criteria for this control group included patients with SCD or a history of stroke. In this prior study, echocardiograms were performed by a standard research protocol and included contrast injections with and without Valsalva maneuver and the studies were centrally reviewed and adjudicated.
Controls
We collected demographics, presence of high-risk moyamoya conditions (including SCD, T21, NF1, Asian descent), age of MM diagnosis, echocardiogram results, clinical history, and laboratory data via review of electronic medical records. For our cases, we recorded the presence of any type of echocardiogram (2D, color Doppler, bubble study with contrast or agitated saline via transthoracic (TTE) or transesophageal (TEE) route), and whether any type of shunting in any direction was observed. Shunting was characterized by the clinical interpretation of the study for the MM patients. Potential right-to-left shunting was defined and categorized as intracardiac shunting (defined by anatomic cardiac shunts or potential intracardiac shunting on Doppler or bubble studies), intrapulmonary shunting (defined by late bubbles on bubble studies, which is the detection of contrast or bubbles in the left atrium or ventricle after 5 cardiac cycles), or no shunting. Potential right-to-left shunting was defined by the identification of any potential shunt, in any direction. Thus, the identification of an ASD or PFO without shunting observed by bubble study or by Doppler, or with identification of left-to-right shunting, was categorized as a potential right-to-left shunt as variations in intracardiac or intrapulmonary pressures (e.g. Valsalva maneuver) could lead to right-to-left shunting and thus “paradoxical embolization” in these patients. Patients who had clinical suggestion of shunting (murmurs, carotid bruits) but without formal diagnosis of a shunt via echocardiogram were not classified as having potential shunts. Patients with documented potential echocardiographic shunting at any time point in their medical records were considered positive shunting, regardless of the temporal relation to MM diagnosis or symptoms.
All control group patients had been enrolled in a prospective study and underwent standardized bubble-contrasted TTEs which included two agitated saline contrast injections with Valsalva maneuver and two agitated contrast saline injections without Valsalva maneuver. Again, potential right-to-left intracardiac shunting was defined by any shunting in any direction by any method; potential intrapulmonary shunting was again defined by late bubbles on bubble studies as above. All TTEs in the control group were reviewed by a central cardiologist in standardized fashion.
The prevalence of potential right-to-left shunting was compared between the two groups using Chi-square statistics. We compared our cases with potential intracardiac shunts, intrapulmonary shunts, and any potential right-to-left shunts to those of the controls. As many of our cases had not undergone contrasted echocardiograms, we performed additional analyses, to evaluate separately those who underwent contrasted studies for the detection of intrapulmonary shunts. We compared the subset of cases with bubble studies with the controls (all of whom had bubble studies). We also compared the individual at-risk groups (those of Asian descent, those with SCD, T21, and NF1) and again compared the prevalence of potential intracardiac, intrapulmonary, and any shunts between these subsets of cases to that of our controls. We used a p-value <0.05 for statistical significance.
Results
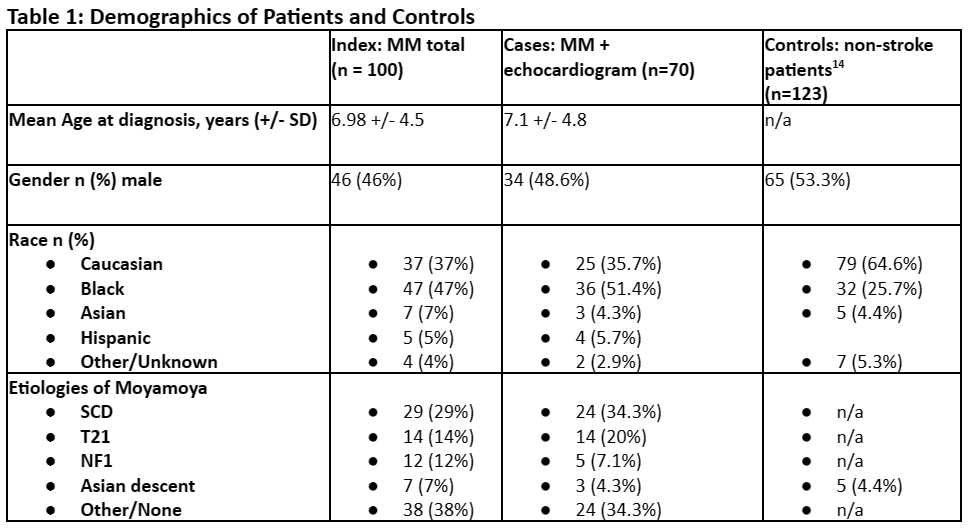
We identified 100 patients in total with moyamoya who fit inclusion criteria for index patients. The age at MM diagnosis ranged from birth to 18.6 years of age, with mean age of 6.9 years, and median age of 6.2 years. Three (3%) did not have age of diagnosis. There were 54 (54%) females and 46 (46%) males. Thirty-seven (37%) were Caucasian, 47 (47%) Black/African American, 7 (7%) Asian, 5 (5%) Hispanic, 4 (4%) other/unknown. Twenty-nine (29%) patients had SCD, 14 (14%) patients had T21, and 12 (12%) patients had NF1. [Table 1, Figure 1]
Of our 100 moyamoya patients, we identified 70 (70%) patients who met inclusion criteria of having MM and echocardiograms, which constituted our cases. In these cases, the age of diagnosis ranged from birth to 18.6 years of age, with a mean age of 7.1 years and a median of 6.2 years. There were 36 (51.4%) females and 34 (48.6%) males. Twenty-five (35.7%) were Caucasian, 36 (51.4%) Black/African American, 3 (4.3%) Asian, 4 (5.7%) Hispanic, 2 (2.9%) other/unknown. Of these 70 patients with MM and echocardiograms, 24 (34.3%) had SCD, 14 (20%) had T21, and 5 (7.1%) had NF1. [Table 1, Figure 1] The etiologies of MM were similar in the total MM group as well as those with MM who had undergone echocardiography. There did not appear to be major group differences between the MM patients who underwent echocardiograms and those who did not. [Figure 1] Tests of significance were not performed between the total MM group and those with MM and echocardiograms.
For our controls, there was no age of diagnosis as these patients were without stroke or MM diagnosis. Fifty-three percent (53%) were male. Sixty-four percent (64%) were Caucasian, while 26% were Black/African American, 4.4% Asian, and 5.3% “other”.14 [Table 1]
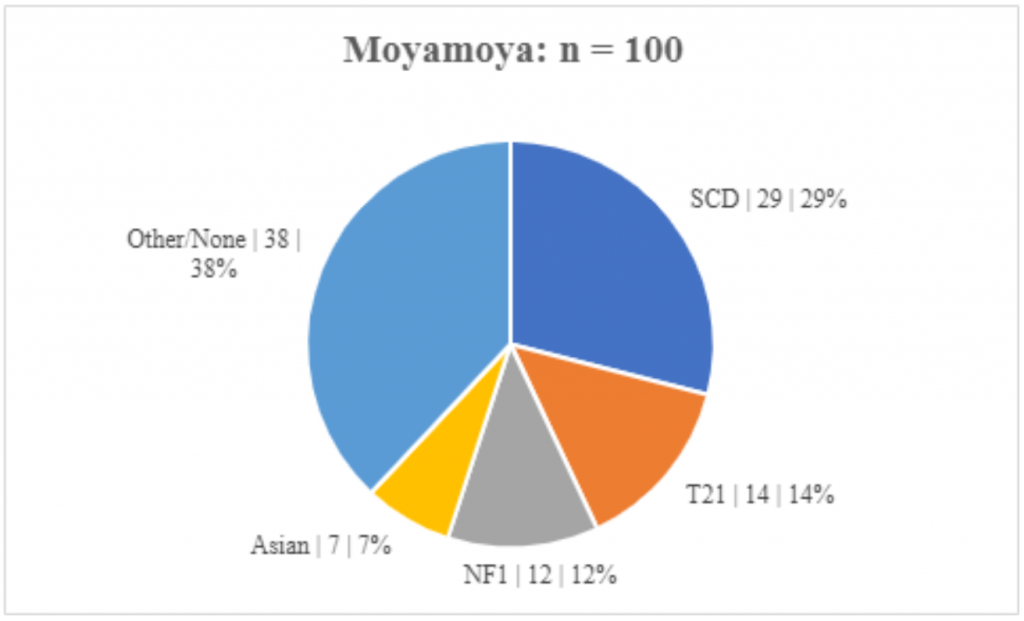
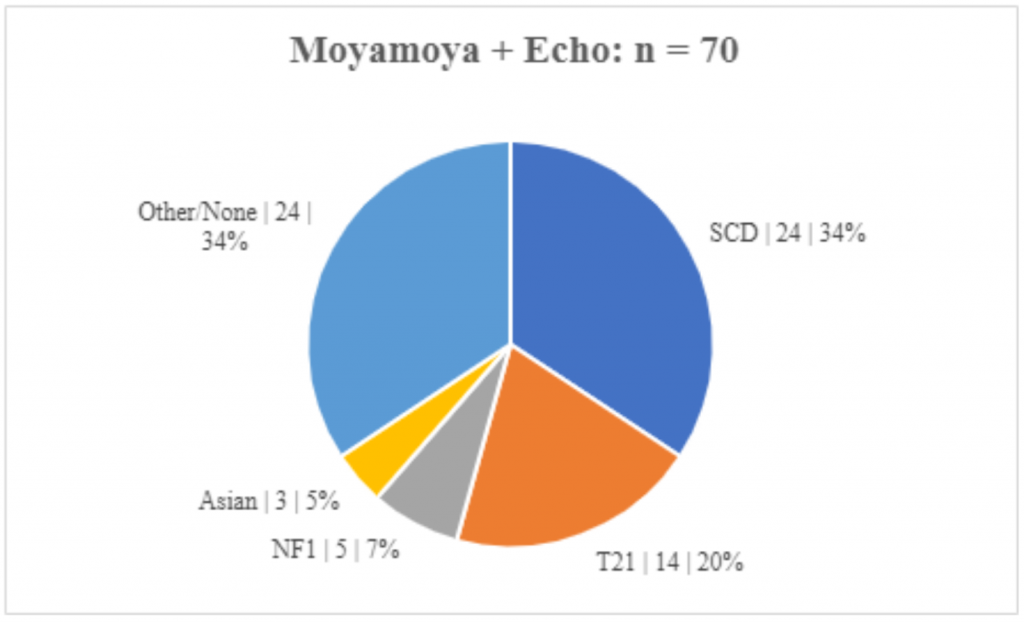
Figure 1. Etiologies of Moyamoya. The etiologies of MM are similar in those with and without echocardiograms. (Subgroup | n | percent)
Prevalence of Shunting
Of our 70 MM cases with echocardiograms, 35/70 (50.0%) had potential shunts observed on echocardiogram. [Figure 2] Among those with any echocardiogram detecting potential shunting, 26 (37.1%) had potential intracardiac shunting. Only 32 of the patients had contrasted studies (bubble echos), and 9 of these 32 (28.1%) showed evidence of potential intrapulmonary shunting. [Table 2] Of our 123 controls, a total of 29 (23.6%) had potential shunts diagnosed on echocardiogram. Twenty-three/123 (18.7%) controls had potential intracardiac shunting while 7/123 (5.7%) had potential intrapulmonary shunting. Using chi-squared statistics, we compared the prevalence of potential shunting between our cases and controls.
We found a significantly higher rate of potential right-to-left shunting reported on clinically-obtained echocardiogram in our patients with MM compared to that identified in a large prospective multicenter research study of echocardiographically-detectable potential shunting in a control group of children without stroke or SCD. Any potential shunting was identified in 35/70 (50%) of MM cases vs 29/123 (23.6%) of controls (p= 0.0001). Potential intracardiac shunting alone was identified in 26/70 (37.1%) of MM cases vs 23/123 (18.7%) of controls (p= 0.004). Intrapulmonary shunting was detected in 9/32 (28.1%) of MM cases with contrasted echocardiograms vs 7/123 (5.7%) of controls (p=0.0002). The study is outlined in Figure 2 with the analysis summarized in Table 2.
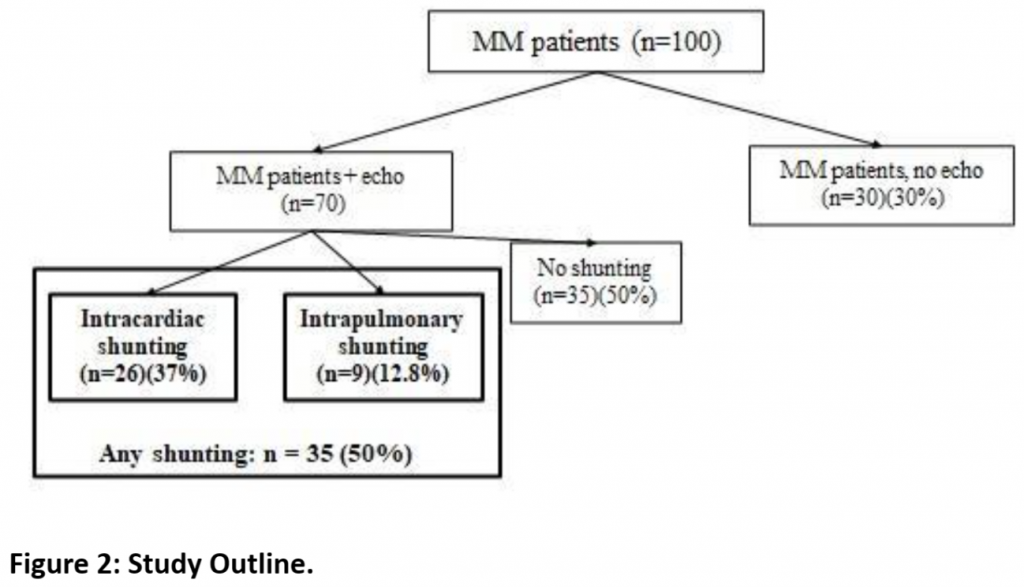

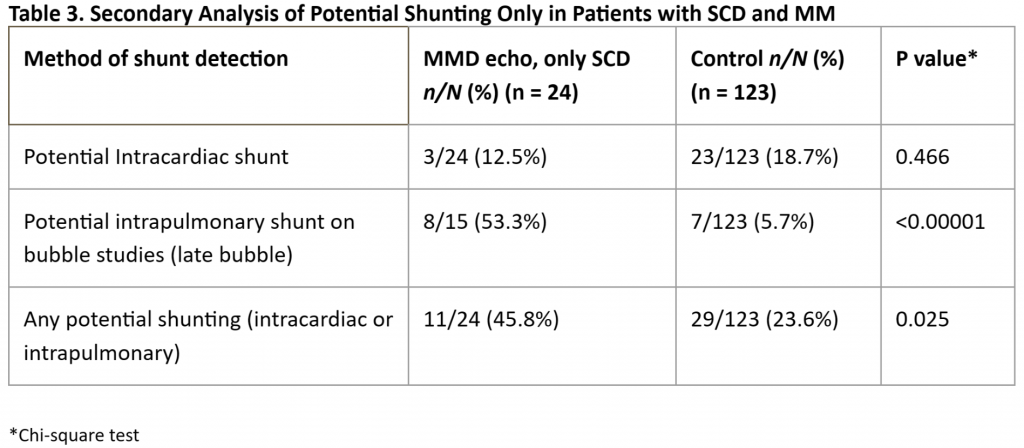
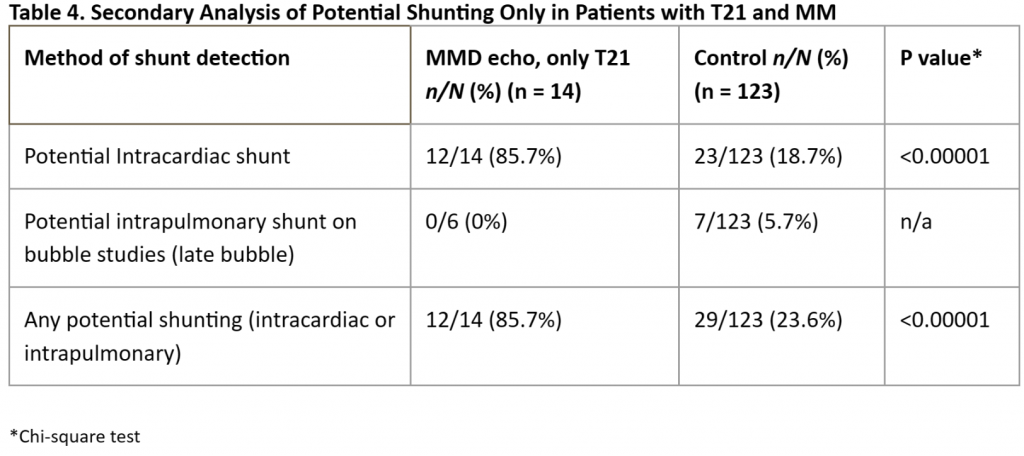
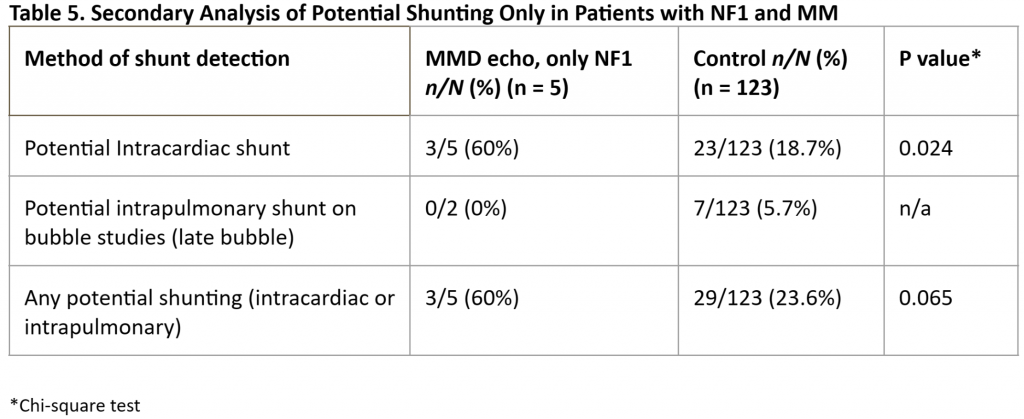
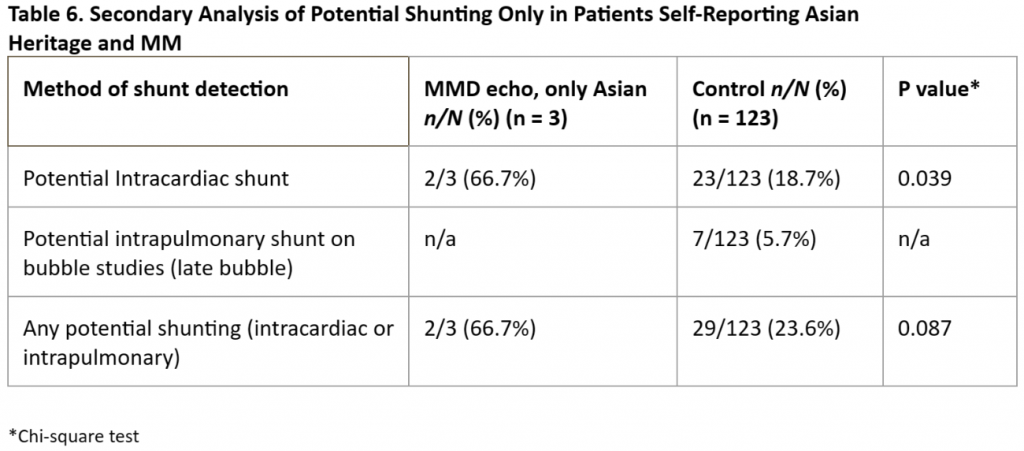
Given the highly significant differences in the prevalence of potential right-to-left shunting in our MM patients vs that in our controls, we performed subanalyses of each individual subgroup but were hampered by small numbers of cases in each group. We found a significantly higher prevalence of “any” potential shunting in MM patients with SCD [Table 3] or T21 [Table 4] vs controls. For patients with MM and NF1 [Table 5] and in our small subset of self-reported Asian patients [Table 6] we found a trend for a higher prevalence of potential shunting compared to that in our controls. Again, these subanalyses were limited by small numbers of cases in each group.
Discussion
Our study, to our knowledge, is the only study evaluating the prevalence of potential right-to-left shunting in children with moyamoya in comparison with a well-studied control group of children without stroke or moyamoya. Stroke rates8,10,14–20 and cardiac shunting prevalence9,15,21–24 have been investigated in patients with SCD, T21, and NF1. We found a significantly higher prevalence of potential right-to-left shunting in our patients with moyamoya compared to a large well studied group of children without stroke or moyamoya.
Moyamoya remains a rare condition with unknown etiology. Most studies theorize multifactorial effects of genetic, inflammatory, or infectious etiologies, contributing to the development of MM. Though the RNF213 gene1,2,12 has been implicated, there is very low penetrance, with only a small percentage of those individuals carrying the mutation developing moyamoya.2,25,26 Other studies have examined the contributions of matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), cytokines, growth factors, and other inflammatory biomarkers affecting the development of MM. 25,27–29 It also remains unclear how these hypothesized etiologies directly affect cerebral vasculature, and why individuals with certain at-risk conditions such as SCD, T21, and NF1 are more likely to develop MMS.
Presumably some of these factors or their modifiers can be inactivated when blood passes through the enzymatically active pulmonary vascular bed.13,30,31 If such factors foster the development of moyamoya in predisposed individuals, then those who experience right-to-left shunting would be at risk of having their cerebral vasculature exposed to higher levels of these active factors via the mechanism of “paradoxical embolization,” which would presumably increase their risk of developing moyamoya vasculopathy. What we do know is that those with SCD do have predisposed poorer lung pathophysiology,32–35 and have higher rates of potential right-to-left shunting, as do those with T21 where atrial and ventricular septal defects are common.36–38
Support for our hypothesis that “vasoactive factors” might affect the cerebral vasculature comes from the evidence from numerous studies of the “vasoactive substance hypothesis” of migraine, where shunting of some venous factors such as 5-hydroxytryptamine, nitric oxide, kinin, and endothelin can bypass and thus avoid metabolism in the pulmonary bed, enter the arterial circulation of the brain and serve as migraine triggers.39,40 Regarding shunting and MM, in a report of 5 children with congenital heart disease (without T21) and early onset moyamoya, 4 of the 5 children had large potential shunts (ventricular septal defect in children with Tetralogy of Fallot) and these 4 were diagnosed with moyamoya by age 2 years. The fifth patient had coarctation and atrial and mitral valve stenosis but no specific mention of potential shunting. Interestingly this last patient was diagnosed with moyamoya later at age 6 years.41 If our hypothesis is correct then one might expect that children with predisposing conditions and larger shunts may present at earlier ages, although we did not see a significant age effect in our group of patients (data not shown).
Unilateral MM is common and can progress to bilateral MM. Few studies specify the lateralization of the affected hemisphere or report which hemisphere is initially or more severely affected. Given that flow patterns from various shunts may direct shunted blood preferentially to one hemisphere, further investigation of the lateralization of the affected hemispheres or the severity of MM may be warranted.
Limitations of our study include the retrospective nature, the lack of echocardiograms in 30 of the 100 patients with diagnosed moyamoya, and that not all echocardiograms in our cases were full Doppler studies or contrasted echocardiograms. Our cases included only echocardiograms obtained for clinical indications. Perhaps unknown indications led to the completion of echocardiograms in those MM patients at increased risk of shunting. However, at our institution, echocardiography is recommended for all patients presenting with stroke. Interestingly, the higher rate of incomplete or non-Doppler echocardiograms without contrast in our cases (as they were clinically obtained studies while our controls were complete contrasted studies done as part of a prospective research study) could bias our analysis AGAINST observing a higher prevalence of potential shunting, as our cases were less stringently evaluated for any potential shunt compared to their control counterparts. Had all our cases undergone echocardiography by the same strict research protocol performed for our controls, we undoubtedly would have observed more cases with some potential shunting. This would have further elevated the high shunting prevalences we observed in the cases. Thus, we may have underestimated the prevalence of potential shunting in our moyamoya group.
Our cases came from a single site in a retrospective review of clinical records, with probable ascertainment bias. Concerns regarding the control group were discussed in the cited study.14 Control patients were deemed “healthy” but had required IV for other medical reasons (scheduled procedures, IV fluids, etc.) due to IRB restrictions; this limited the number of patients that were available, but we do not see how this could have biased our results towards detection of fewer shunts. Some potential shunts, such as PFO, ASD, or VSD can close spontaneously in early childhood. Thus, our echocardiograms performed at later ages, after the development of MM in our cases, would have failed to detect potential shunting that could have been present at a younger age. This, too, would bias our study against identification of a higher prevalence of potential shunting in MM cases.
An additional concern is that our data could possibly have been biased towards patients with at-risk conditions given their underlying comorbidities – for instance, did we have a statistically significant result because we had a biased sample of cases with poor lung and/or cardiac pathology. However, our subanalyses of each respective at-risk group remained significant or trended towards significance irrespective of subcategory, suggesting this bias likely played a small role, if any. Finally, we recognize that the presence of a potential shunt does not directly cause right-to-left shunting. We base the potential or possibility of said shunting based on understanding of pathophysiology, and “paradoxical” embolization in situations that increase pulmonary pressures such as Valsalva, infections, acute chest syndrome (in SCD patients), etc.
In conclusion, we observed a statistically significant increased prevalence of potential intracardiac, intrapulmonary, and total (any) potential right-to-left shunting in our patients with moyamoya and echocardiograms compared to our control group of non-stroke patients without moyamoya. While we cannot conclude that shunting causes the development of moyamoya from such a case-control study, the data support our hypothesis that moyamoya development in predisposed populations may relate, in part, to unknown factors avoiding enzymatic inactivation in the lungs, possibly via shunting / paradoxical embolization. These factors may thereby travel to the central nervous system to trigger cerebral arteries to develop moyamoya in these predisposed populations. These observations have potential mechanistic and therapeutic implications for moyamoya. This will need to be verified in larger prospective studies.
Acknowledgements
The authors thank Fenella J Kirkham for vigorous discussion and inspiration.
Conflict of Interest
The authors declare no potential conflicts of interest. MM Dowling recused himself from editorial decision making on this manuscript. Editorial decisions were made by the Associated Editors for this manuscript.
References
- Lee S, Rivkin MJ, Kirton A, DeVeber G, Elbers J. Moyamoya Disease in Children: Results from the International Pediatric Stroke Study. J Child Neurol. 2017;32(11). doi:10.1177/0883073817718730
- Ihara M, Yamamoto Y, Hattori Y, et al. Moyamoya disease: diagnosis and interventions. Lancet Neurol. 2022;21(8):747-758. doi:10.1016/S1474-4422(22)00165-X
- Berry JA, Cortez V, Toor H, Saini H, Siddiqi J. Moyamoya: An Update and Review. Cureus. 2020;12(10). doi:10.7759/CUREUS.10994
- Kuroda S. Asymptomatic Moyamoya Disease: Literature Review and Ongoing AMORE Study. Neurol Med Chir (Tokyo). 2015;55(3):194-198. doi:10.2176/nmc.ra.2014-0305
- Ma J, Liu Y, Ma L, Huang S, Li H, You C. RNF213 polymorphism and Moyamoya disease: A systematic review and meta-analysis. Neurol India. 2013;61(1):35-39. doi:10.4103/0028-3886.107927
- Abdelgadir A, Akram H, Dick MH, et al. A Better Understanding of Moyamoya in Trisomy 21: A Systematic Review. doi:10.7759/cureus.23502
- Newman S, Boulter JH, Malcolm JG, Pradilla I, Pradilla G. Outcomes in Patients with Moyamoya Syndrome and Sickle Cell Disease: A Systematic Review. doi:10.1016/j.wneu.2019.11.137
- Barreto-Duarte BI, Andrade-Gomes FH, Arriaga MB, Araú jo-Pereira M, Manuel Cubillos-Angulo J, AndradeID BB. Association between neurofibromatosis type 1 and cerebrovascular diseases in children: A systematic review. Published online 2021. doi:10.1371/journal.pone.0241096
- Amlie-Lefond C, Bernard TJ, Sébire G, et al. Predictors of Cerebral Arteriopathy in Children With Arterial Ischemic Stroke. Circulation. 2009;119(10):1417-1423. doi:10.1161/CIRCULATIONAHA.108.806307
- Dowling MM, Lee N, Quinn CT, et al. Prevalence of Intracardiac Shunting in Children with Sickle Cell Disease and Stroke. Journal of Pediatrics. 2010;156(4):645-650. doi:10.1016/j.jpeds.2009.10.012
- Lauzier DCB, Guilliams KPMM, Kansagra AMM. Imaging and epidemiology of Moyamoya Vasculopathy: Case report and brief review. Pediatric Stroke Journal. 2022;4:16-32. Accessed May 7, 2023. https://pediatricstrokejournal.com/imaging-and-epidemiology-of-moyamoya-vasculopathy-case-report-and-brief-review/
- Wang Y, Zhang Z, Wei L, et al. Predictive role of heterozygous p.R4810K of RNF213 in the phenotype of Chinese moyamoya disease. Neurology. 2020;94(7):e678-e686. doi:10.1212/WNL.0000000000008901
- Said SI. Circulation Research – Brief Reviews: Metabolic Functions of the Pulmonary Circulation. Published online 1982. Accessed May 7, 2023. http://ahajournals.org
- Dowling MM, Quinn CT, Ramaciotti C, et al. Increased prevalence of potential right-to-left shunting in children with sickle cell anaemia and stroke. Br J Haematol. 2017;176(2):300-308. doi:10.1111/bjh.14391
- Razdan S, Strouse JJ, Naik R, et al. Patent Foramen Ovale in Patients with Sickle Cell Disease and Stroke: Case Presentations and Review of the Literature. Case Rep Hematol. 2013;2013:1-5. doi:10.1155/2013/516705
- Cramer SC, Robertson RL, Dooling EC, Scott RM. Moyamoya and Down Syndrome. Stroke. 1996;27(11):2131-2135. doi:10.1161/01.STR.27.11.2131
- Sobey CG, Judkins CP, Sundararajan V, Phan TG, Drummond GR, Srikanth VK. Risk of Major Cardiovascular Events in People with Down Syndrome. Published online 2015. doi:10.1371/journal.pone.0137093
- Dowling MM, Hynan LS, Lo W, et al. International Paediatric Stroke Study: stroke associated with cardiac disorders. doi:10.1111/j.1747-4949.2012.00925.x
- Sinclair MBChB AJ, Fox CK, Ichord RN, et al. Stroke in Children With Cardiac Disease: Report From the International Pediatric Stroke Study Group Symposium. Published online 2015. doi:10.1016/j.pediatrneurol.2014.09.016
- Dowling MM, Ikemba CM. Intracardiac shunting and stroke in children: a systematic review. J Child Neurol. 2011;26(1):72-82. doi:10.1177/0883073810383913
- Morrison ML, McMahon CJ, Morrison ML, McMahon CJ. Congenital Heart Disease in Down Syndrome. Advances in Research on Down Syndrome. Published online January 31, 2018. doi:10.5772/INTECHOPEN.71060
- Delany DR, Gaydos SS, Romeo DA, et al. Down syndrome and congenital heart disease: perioperative planning and management. Journal of Congenital Cardiology 2021 5:1. 2021;5(1):1-14. doi:10.1186/S40949-021-00061-3
- Saccocci M, Ferraro F, Blasi S, et al. First Case of Tricuspid Valve Surgery in a Neurofibromatosis Type 1 Patient. Heart Views. 2021;22(3):214. doi:10.4103/HEARTVIEWS.HEARTVIEWS_17_21
- Pinna V, Daniele P, Calcagni G, et al. Prevalence, Type, and Molecular Spectrum of NF1 Mutations in Patients with Neurofibromatosis Type 1 and Congenital Heart Disease. Genes (Basel). 2019;10(9). doi:10.3390/GENES10090675
- Mertens R, Graupera · M, Gerhardt · H, et al. The Genetic Basis of Moyamoya Disease. 2022;13:25-45. doi:10.1007/s12975-021-00940-2
- Asselman C, Hemelsoet D, Eggermont D, Dermaut B, Impens F. Moyamoya disease emerging as an immune-related angiopathy. Trends Mol Med. 2022;28(11):939-950. doi:10.1016/J.MOLMED.2022.08.009
- Dlamini N, Muthusami P, Amlie-Lefond C. Childhood Moyamoya: Looking Back to the Future. Pediatr Neurol. 2018;91:11-19. doi:10.1016/j.pediatrneurol.2018.10.006
- Fullerton HJ, Deveber GA, Hills NK, et al. Inflammatory Biomarkers in Childhood Arterial Ischemic Stroke: Correlates of Stroke Etiology and Recurrence. Stroke; a journal of cerebral circulation. 2016;47(9):2221. doi:10.1161/STROKEAHA.116.013719
- Liu W, Sun J, Shi Z, et al. Circulating Inflammatory Cytokine Associated with Poor Prognosis in Moyamoya Disease: A Prospective Cohort Study. J Clin Med. 2023;12(3). doi:10.3390/JCM12030823
- Alvarado A, Arce I. Metabolic Functions of the Lung, Disorders and Associated Pathologies. J Clin Med Res. 2016;8(10):689-700. doi:10.14740/JOCMR2668W
- De Wet C, Moss J. Metabolic Functions of the Lung. Anesthesiol Clin North Am. 1998;16(1):181-199. doi:10.1016/S0889-8537(05)70013-4
- Minter KR, Gladwin MT. Pulmonary complications of sickle cell anemia: A need for increased recognition, treatment, and research. Am J Respir Crit Care Med. 2001;164(11). doi:10.1164/ajrccm.164.11.2104101
- Khoury RA, Musallam KM, Mroueh S, Abboud MR. Pulmonary complications of sickle cell disease. Hemoglobin. 2011;35(5-6). doi:10.3109/03630269.2011.621149
- Miller AC, Gladwin MT. Pulmonary complications of sickle cell disease. Am J Respir Crit Care Med. 2012;185(11). doi:10.1164/rccm.201111-2082CI
- Siddiqui AK, Ahmed S. Pulmonary manifestations of sickle cell disease. Postgrad Med J. 2003;79(933). doi:10.1136/pmj.79.933.384
- Bush D, Galambos C, Ivy DD, Abman SH, Wolter-Warmerdam K, Hickey F. Clinical Characteristics and Risk Factors for Developing Pulmonary Hypertension in Children with Down Syndrome. Journal of Pediatrics. 2018;202. doi:10.1016/j.jpeds.2018.06.031
- Irving CA, Chaudhari MP. Cardiovascular abnormalities in Down’s syndrome: Spectrum, management and survival over 22 years. Arch Dis Child. 2012;97(4). doi:10.1136/adc.2010.210534
- Santoro SL, Steffensen EH. Congenital heart disease in Down syndrome – A review of temporal changes. Journal of Congenital Cardiology. 2021;5(1). doi:10.1186/s40949-020-00055-7
- Cao W, Shen Y, Zhong J, Chen Z, Wang N, Yang J. The Patent Foramen Ovale and Migraine: Associated Mechanisms and Perspectives from MRI Evidence. Brain Sci. 2022;12(7). doi:10.3390/brainsci12070941
- Wilmshurst P, Nightingale S. The role of cardiac and pulmonary pathology in migraine: A hypothesis. Headache. 2006;46(3):429-434. doi:10.1111/j.1526-4610.2006.00374.x
- Lutterman J, Scott M, Nass R, Geva T. Moyamoya Syndrome Associated With Congenital Heart Disease. Published online 1998. Accessed November 11, 2022. http://publications.aap.org/pediatrics/article-pdf/101/1/57/823714/57.pdf
Increased Prevalence of Potential Right to Left Shunting