Original Research
1Purva R. Choudhari, 2Mathew Stokes, 3Cory M. Pfeifer, 4Andrew D. Hershey, 1Tonia Sabo
1 Division of Pediatric Hospital Medicine, Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, Texas
2 Division of Pediatric Neurology, Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, Texas
3 Department of Radiology, University of Texas Southwestern Medical Center, Dallas, Texas
4 Department of Pediatrics and Neurology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio
Corresponding Author:
Tonia Sabo, MD
Associate Professor
Department of Pediatrics, Divisions of Neurology and Pain Management
UT Southwestern Medical Center
tonia.sabo@utsouthwestern.edu
Copyright © belongs to author(s)
All rights reserved.
Any redistribution or reproduction of part or all of the contents in any form is prohibited other than the following:
- you may print or download to a local hard disk extracts for your personal and non-commercial use only
- you may copy the content to individual third parties for their personal use, but only if you acknowledge the website as the source of the material
You may not, except with our express written permission, distribute or commercially exploit the content. Nor may you transmit it or store it in any other website or other form of electronic retrieval system.
Abstract
Hemiplegic migraine is a rare form of migraine with aura that presents with reversible hemiparesis as part of the aura. In some cases, it can result in significant neurological co-morbidities, including stroke. We present a case of radiographically proven cerebral artery vasospasm leading to stroke in an adolescent patient with hemiplegic migraine. He presented with right-sided hemiparesis, aphasia, and encephalopathy after stopping his preventive therapy (topiramate). After seventy-two hours without improvement using traditional migraine acute therapies, brain imaging demonstrated a left middle cerebral artery territory infarct and vasospasm in the right middle cerebral and anterior cerebral arteries. The calcium channel blocker verapamil resulted in improvement in both clinical symptoms and radiographic vasoconstriction. Employing a calcium channel blocker as his maintenance therapy was guided by his underlying CACNA1A gene mutation. This case involves an unusual presentation of hemiplegic attack and stroke resulting from vasospasm and is one of a few pediatric hemiplegic migraine cases with improvement of stroke-related deficits with calcium channel blocker.
Introduction
Hemiplegic migraine (HM) is a rare form of migraine with an aura that includes reversible hemiparesis in addition to typical aura such as sensory, speech, and vision changes.1 Patients with afflicted first or second-degree relatives meet the International Classification of Headache Disorders, 3rd edition (ICHD-3)’s diagnostic criteria for familial hemiplegic migraine (FHM); those without relatives with HM carry the diagnosis of sporadic hemiplegic migraine (SHM).1
FHM is linked with mutations in three known gene loci but other rare pathogenic variants have been associated with HM, suggesting that other genes may be involved. FHM-1 patients have missense or gain-of-function mutations in the CACNA1A gene. The CACNA1A gene at chromosome 19p13 codes for the alpha subunit of the Cav2.1 channel, a P/Q type calcium channel.2 CACNA1A mutations also result in episodic ataxia 2 (EA-2) via loss of function mutations and in spinocerebellar ataxia type 6 (SCA-6) via repeat expansions. While EA-2 and SCA-6 are predominantly characterized by ataxia, EA-2 patients can have migraine and SCA-6 patients can experience headaches and nausea that are suggestive of migraine.3
Mutations in ATP1A2, which codes for Na-K ATPase, have been identified in patients with FHM-2, while mutations in SCN1A correlate with FHM-3.2 However, 25% of patients with FHM do not have mutations associated with these three genes. A fourth FHM gene has not yet been identified, but PRRT2, mutations of which result in paroxysmal dyskinesias and childhood epilepsies, has been suggested as the fourth gene. As research continues, FHM has also been identified in patients with mutations in PNKD, the gene implicated in paroxysmal non-kinesigenic dyskinesia, and SLC2A1, the glucose transporter protein type 1 gene.3
While traditional migraine preventives have been employed to treat HM, as well as carbonic anhydrase inhibitors, some treatments have been targeted towards the specific HM mutation. Capitalizing on the efficacy of acetazolamide (a carbonic anhydrase inhibitor) in EA-2, this medication has prevented migraine in specific FHM-1 patients.2 Calcium channel blockers have been used successfully as acute and preventive treatments in patients with CACNA1A mutations, as described in several case reports.2, 4, 5
We present a case of radiographically proven cerebral artery vasospasm leading to stroke in an adolescent patient experiencing SHM-1 that improved with calcium channel blocker therapy. We will review the evaluation of patients with headache and hemiparesis, the genetics and features of SHM-1, and treatment approaches for SHM-1.
Clinical Case
Our patient is a left-handed 16-year-old African-American male who initially presented to the concussion clinic four days after injury with persistent post-traumatic headache and altered cognitive abilities, causing poor school performance. Past medical history revealed a pre-injury history of monthly headaches, consisting of occipital throbbing, and one occasion of nausea and dizziness accompanying a headache, which led to a fall and the most recent concussion as a result of the fall. He was physically active, playing football, and reported three previous concussions; this most recent concussion occurred at age 13 years. After resolution of concussive symptoms, which correlated with his improving physical exam, he continued to be seen in clinic for new episodes of reversible left arm numbness and “heaviness” after onset of severe, bi-frontal, throbbing headaches. Sometimes, bilateral lower extremity weakness accompanied the headaches. Clinical examinations revealed deficits that had not been present during prior visits, including delayed short-term and long-term recall, mildly ataxic speech, and abnormal cerebellar function, including abnormal finger-to-nose and tandem gait. Magnetic resonance imaging (MRI) of the brain revealed mild superior cerebellar vermian and bi-hemispheric atrophy, and magnetic resonance angiography (MRA) of the head and neck was normal. Consequently, a diagnosis of SHM was suspected, prompting transition to topiramate, which improved the frequency and severity of attacks. The teenager has no known family history of FHM. Identified triggers for attacks included minor head trauma. He was diagnosed with SHM after genetic testing, using our hospital’s devised gene panel, revealed a likely pathogenic variant, Ile1714Thr, of the CACNA1A gene.6 He was advised to halt contact sports; however, he was then lost to follow-up.
One year after the diagnosis, he developed a prolonged hemiplegic attack necessitating hospital admission. At this time, he was no longer taking his topiramate for prevention. His headache had begun two days prior to admission with right upper extremity weakness, as was typical for his hemiplegic attacks. Despite taking ibuprofen, later in the day, symptoms had progressed to right hemiplegia and dysphasia. During the admission exam, he was lethargic and mute and had right hemiplegia and moderate headache. Symptoms progressed to moderate encephalopathy, bilateral arm and leg weakness (right greater than left), right facial asymmetry, dysphagia, dysconjugate gaze, and continued aphasia, within twenty-four hours of admission. The reinstitution of topiramate and use of acute headache therapies were ineffective. Seventy-two hours after the onset of symptoms, MRI brain demonstrated an acute left parieto-occipital infarct (Figure 1A.1). MRA head revealed mild diffuse narrowing of the left middle cerebral artery (L MCA) (Figure 1A.2). Initial flow velocities on transcranial doppler (TCD) were elevated in the right MCA and anterior cerebral artery (R ACA) as well, suspicious for vasospasm.
Based on the MRI findings, a detailed workup ensued, including CSF cell counts and differential and HSV PCR, CSF autoimmune encephalitis panel, inflammatory markers, thyroid studies, and hypercoagulability labs. Performing a lumbar puncture was carefully considered and ultimately decided upon in light of the teenager’s persistent encephalopathy and to assess for infectious and autoimmune causes of encephalitis and for vasculitis. The majority of testing returned unremarkable, except for an elevated opening pressure of 50 cm H2O demonstrated by lumbar puncture manometry, likely due to cerebral edema. Continuous electroencephalogram (EEG) demonstrated left focal continuous polymorphic delta slowing, consistent with his left sided infarct.
The persistence of profound encephalopathy and weakness, as well as concern for cerebral vasospasm, prompted a change in treatment strategy. A calcium channel blocker, verapamil, was instituted. Radiographically, he was monitored with serial MRI/MRA brain and TCD every one to three days until both studies trended improvement of cerebral vasospasm (Figure 1B, D).
Two weeks after admission, when the last neuroimaging was performed, he was able to follow some simple commands slowly, conflated some words, had limited facial movements, had improved right sided strength at 4/5, and had ataxic gait. A few days later when he was discharged, he had increased verbalization, increased spontaneous facial movements, and had regained full strength. However, motor slowness, right sided hyperreflexia, and ataxic gait persisted. He was discharged home on two headache preventives: verapamil 120mg daily and long-acting topiramate 100 mg daily, and on daily aspirin.
An increase in verapamil to 240mg daily occurred one-month post-discharge, due to concern for continued vasospasm and chronic ischemia as revealed by follow-up MRA and MRI brain. After one month of increased calcium channel blocker therapy, repeat neuroimaging demonstrated left brain hemi-atrophy, resolution of restricted diffusion, and improved left MCA caliber (Figure 1C, D). During this two-month follow-up visit, he demonstrated marked improvement but still exhibited mild to moderate deficits with speech articulation, word retrieval, short-term memory and comprehension. He has had two hemiplegic attacks since the hospitalization.
Discussion
For any patient with prolonged headache and hemiparesis, HM would be considered a diagnosis of exclusion after other critical etiologies, such as seizure, stroke, reversible cerebral vasoconstriction syndrome (RCVS), demyelinating disorders, and metabolic, toxic, and neoplastic disorders, have been ruled out. Focused workup would be dependent upon associated medical history and presentation.2 In athletes with recent mild traumatic brain injury (TBI), headache is a common symptom and can be severe. However, the presence of focal findings, such as unilateral motor weakness, would be considered a “red flag” and prompt urgent workup to rule out intracranial and cervical spine complications.8
Our patient initially presented to clinic with protracted headache and other post-concussive symptoms, followed by hemiplegic attacks months later. In addition, cerebellar atrophy on MRI brain as well as the patient’s ataxic speech and tremor prompted the initial concern for SHM and gene testing. The neuro-genetic diagnosis provided valuable information for clinical care during his hospitalization for HM-related stroke.
The core feature of HM is motor weakness, which must be reversible but can last up to a week.1 In addition to the motor aura, the most common aura symptoms include hemisensory symptoms, positive or negative visual symptoms, aphasia (predominantly expressive), and basilar symptoms (clumsiness, vertigo).2 A very careful examination must be performed to differentiate the relatively common sensory aura from the rare HM. HM-1 patients can experience cerebellar symptoms, including nystagmus, ataxia, and dysarthria, and such patients may have chronic cerebellar atrophy. Neurologic co-morbidities include epilepsy, intellectual disability, and stroke.2 The CACNA1A mutation is significant in that minor head trauma can provoke a severe HM attack, resulting in cerebral edema, stroke, and potentially coma.2
Over thirty CACNA1A mutations have been identified in FHM-1 patients, with Thr666Met as the most frequent mutation.2 Of patients with SHM, the frequency of variants in the three main FHM genes is low.2 This has often raised the concern of whether SHM is a different disorder than FHM and requires further careful evaluation.9 Our patient, who did not have any relatives with FHM, was heterozygous for a missense mutation that substituted threonine for isoleucine (reported as c.5141T>C, Ile1714Thr). His mutation was considered as likely pathogenic due to similar variant in previous literature.6 A similar variant (referred to as c.5404 T>C in the article) was reported as a de novo mutation in a Swedish proband, who had FHM and ataxia, and her two children with FHM, ataxia, and seizures.6
Persistence of suspected HM symptoms for longer than twenty-four hours or lack of significant improvement in symptoms with traditional migraine management should prompt neuroimaging to rule out cerebral infarction or vasospasm. MRI brain at the time of HM presentation may be normal or demonstrate only cerebral edema. However, follow-up imaging can reveal changes in diffusion weight imaging (DWI) and apparent diffusion coefficient (ADC), indicating increased restricted diffusion (as illustrated in our case) and cerebral ischemia.4, 10 Our case highlights the importance of cerebral vessel imaging (MRA) to evaluate for vasospasm. Additionally, functional vascular testing with TCD for regular monitoring of vasospasm is advantageous in that it is a non-invasive, bedside imaging modality that does not require the use of contrast or gadolinium.
Vasospasm has been previously theorized to be present during an HM episode, and our case supports this concept for CACNA1A hemiplegic migraine.11 An intriguing thought is that CACNA1A hemiplegic migraine could be related to RCVS. RCVS can present with recurring, acute-onset, rapidly escalating headache associated with nausea, photophobia, and phonophobia. Ischemia can occur, typically after a week, in a watershed distribution.7 The characteristic pattern on vessel imaging is that of “beads on string” because vessel constriction is interspersed with dilation. In the acute setting, patients with RCVS receive oral or intravenous calcium channel blockers, and intra-arterial therapy with nimodipine may be necessary for severe cases.7 After his hospitalization, our patient demonstrated chronic ischemia, which was attributed to his persistent vasoconstriction, that reversed with increase in calcium channel blocker. This is an interesting parallel to patients with RCVS who can have continued vasoconstriction after the sudden, severe headache and require 4-12 weeks of oral medication, typically calcium channel blockers, in an attempt to normalize vessel caliber with variable success.7
Management of HM has been based on small studies and case reports.2 Knowledge of the causative mutation can guide treatment selection. Calcium channel blockers have been used successfully as acute and preventive headache therapies in patients with CACNA1A mutations, even terminating the HM attack when given days after traditional therapies failed.2, 4, 5 Intravenous verapamil led to complete reversal of stroke symptoms in a pediatric patient with CACNA1A mutation following administration shortly after presentation.4 This patient, at one-year follow-up, had not had future HM episodes or strokes while on oral maintenance verapamil.4 Similarly, our patient has had a significant decrease in the frequency of his HM episodes and no strokes while he has been on daily verapamil. Knowing our patient’s CACNA1A gene mutation supported the selection of calcium channel blockers as treatment.
However, calcium channel blockers may not be beneficial in HM patients without mutations in calcium channels as nimodipine worsened a hemiplegic attack in a FHM-2 patient.12 Alternative management options include the carbonic anhydrase inhibitor, acetazolamide, which has been used as a preventive in a family of patients with FHM-1 as well as an abortive to alleviate hemiparesis in a pediatric patient with FHM-1.13 Intranasal ketamine, targeting NMDA glutamate receptors, was efficacious as acute therapy in nearly half of FHM patients in one case series.2 The use of triptans has been considered contraindicated due to concern for vasoconstriction but a retrospective study of HM patients revealed triptans to be a safe option for those patients.2
This case highlights the risks associated with HM-1, which in this patient resulted in permanent deficits from completed ischemia. Although hemiparesis can extend past 24 hours1, other protracted symptoms in an HM patient should prompt further investigation, including brain and vascular imaging with strong consideration given to obtaining neuroimaging during the initial day of the patient’s clinical course. Our patient’s prolonged symptoms and subsequent imaging revealed his cerebral infarct and cerebral vasospasm, facilitating targeted management. Early use of calcium channel blocker, particularly in HM patients with known CACNA1A mutations, may mitigate an HM attack and prevent future attacks. We suggest that CACNA1A-related hemiplegic migraine can be associated with vasospasm as our patient experienced vasospasm and resulting cerebral ischemia. This bolsters the role for calcium channel blockers in this HM population.
References
- Headache classification committee of the international headache society (ihs) the international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38:1-211
- Russell MB, Ducros A. Sporadic and familial hemiplegic migraine: Pathophysiological mechanisms, clinical characteristics, diagnosis, and management. The Lancet Neurology. 2011;10:457-470
- Sutherland HG, Albury CL, Griffiths LR. Advances in genetics of migraine. J Headache Pain. 2019;20:72
- Knierim E, Leisle L, Wagner C, Weschke B, Lucke B, Bohner G, et al. Recurrent stroke due to a novel voltage sensor mutation in cav2.1 responds to verapamil. Stroke. 2011;42:e14-17
- Yu W, Horowitz SH. Treatment of sporadic hemiplegic migraine with calcium-channel blocker verapamil. Neurology. 2003;60:120-121
- Kors EE, Melberg A, Vanmolkot KRJ, Kumlien E, Hann J, Raininko R, et al. Childhood epilepsy, familial hemiplegic migraine, cerebellar ataxia, and a new cacna1a mutation. Neurology. 2004;63
- Qubty W, Irwin SL, Fox CK. Review on the diagnosis and treatment of reversible cerebral vasoconstriction syndrome in children and adolescents. Semin Neurol. 2020;40:294-302
- Pinchefsky E, Dubrovsky AS, Friedman D, Shevell M. Part i–evaluation of pediatric post-traumatic headaches. Pediatr Neurol. 2015;52:263-269
- Black DF. Sporadic hemiplegic migraine. Current Pain and Headache Reports. 2004;8:223-228
- Kedia S, Stence N, Manco-Johnson M, Armstrong-Wells J, Bernard TJ. Late cytotoxic edema in 2 children with hemiplegia: Hemiplegic migraine or stroke? Headache. 2012;52:674-678
- Safier R, Cleves-Bayon C, Vaisleib I, Siddiqui A, Zuccoli G. Magnetic resonance angiography evidence of vasospasm in children with suspected acute hemiplegic migraine. J Child Neurol. 2014;29:789-792
- Mjaset C, Russell MB. Intravenous nimodipine worsening prolonged attack of familial hemiplegic migraine. J Headache Pain. 2008;9:381-384
- Omata T, Takanashi J, Wada T, Arai H, Tanabe Y. Genetic diagnosis and acetazolamide treatment of familial hemiplegic migraine. Brain Dev. 2011;33:332-334
| Key Points |
| Three genes are linked with hemiplegic migraine, CACNA1A, ATPA2, and SCN1A, in addition to a suspected fourth gene. |
| The key feature of hemiplegic migraine is reversible weakness. Typical aura symptoms, including sensory, speech, and/or vision changes, can also occur. |
| Protracted HM symptoms should prompt further investigation including brain/vascular imaging as stroke resulting in permanent deficits is a possible significant consequence. |
| Calcium channel blockers are efficacious acute and preventive treatments for CACNA1A-related hemiplegic migraine. |
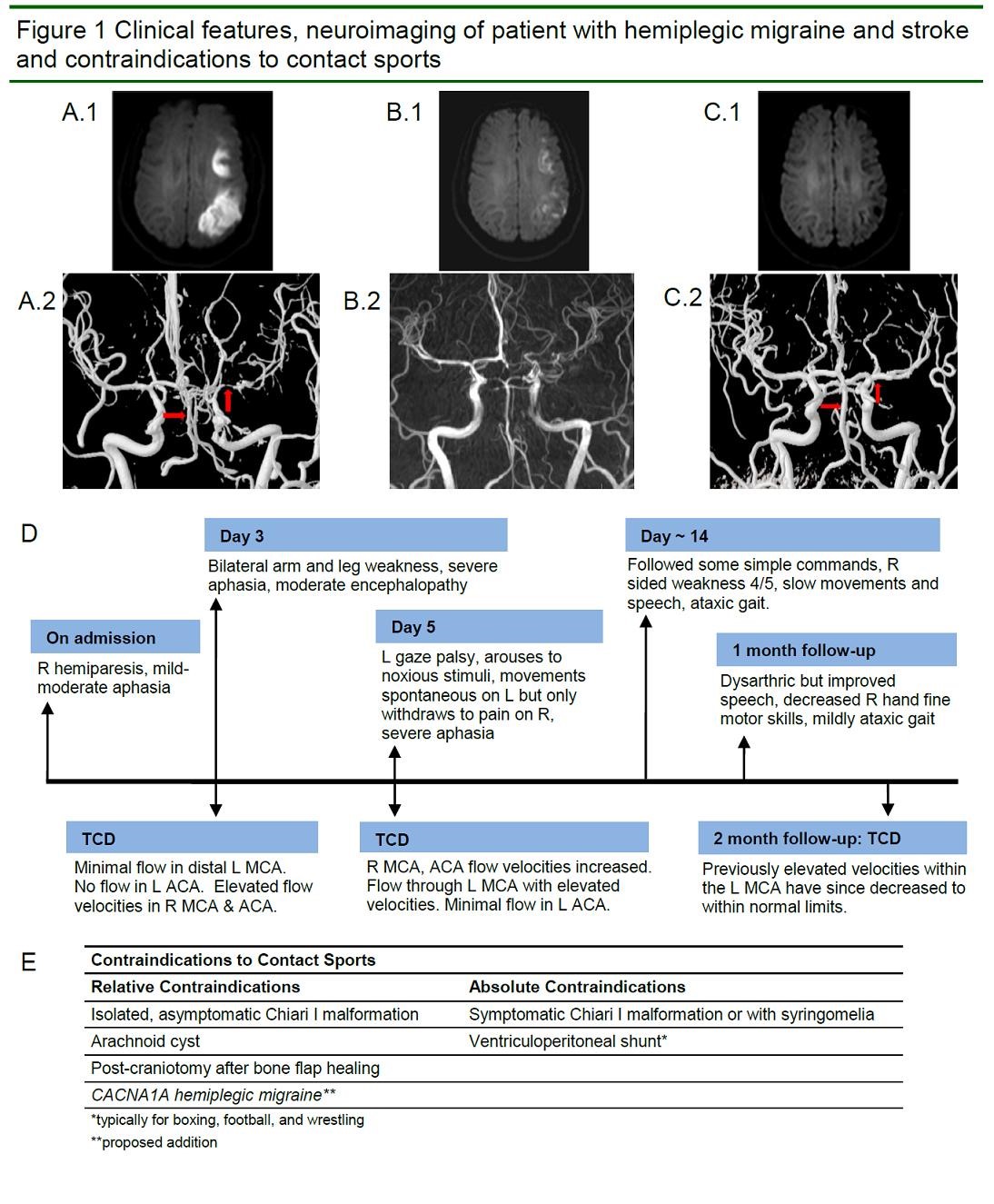
(A.1) Axial DWI of the MRI brain on Day 3 of symptoms reveals diffusion restriction in the left posterior parieto-occipital cortex. (A.2) MRA of the brain also obtained on Day 3 of symptoms demonstrates vasospasm of the left MCA and basilar artery (arrows). (B.1) Axial DWI at ~ 2 weeks demonstrates evolving left MCA distribution infarct. (B.2) MRA brain shows improved left MCA caliber. (C.1) DWI of the brain at two-month follow-up (~ 10 weeks after symptoms onset) shows left parieto-occipital encephalomalacia and no diffusion restriction. (C.2) MRA shows improved left MCA caliber after increase in calcium channel blocker (arrows). (D) Timeline of clinical symptoms and TCD findings from admission to follow-up. R = right. L = left. DWI = diffusion-weighted imaging. MRI = magnetic resonance imaging. MRA = magnetic resonance angiography. MCA = middle cerebral artery. TCD = transcranial doppler.
Challa Case Report