Case Report
Authors: Richard B Carozza, MD, MS1, Eric Dornoff, MD1, Asha Sarma, MD2, Lori C Jordan, MD, PhD1, Matthew Hiller, MD1
1 Department of Pediatrics, Division of Pediatric Neurology, Vanderbilt University Medical Center, Nashville, TN, USA
2 Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, USA
Corresponding author
Richard B. Carozza, MD, MS
Department of Pediatrics, Division of Pediatric Neurology
Vanderbilt University Medical Center
2200 Children’s Way, DOT 9
Nashville, TN 37232
Phone: 615-936-5536
Fax: 615-936-8094
Email: richard.carozza@gmail.com
Key Words: Reversible cerebral vasoconstriction syndrome, posterior reversible encephalopathy syndrome, cerebral edema, endothelial dysfunction, cerebral autoregulation, seizure
Abbreviations: reversible cerebral vasoconstriction syndrome (RCVS), posterior reversible encephalopathy syndrome (PRES), computed tomography (CT), computed tomography angiogram (CTA), middle cerebral artery (MCA), posterior cerebral artery (PCA), magnetic resonance imaging (MRI), magnetic resonance angiogram (MRA), diffusion weighted imaging (DWI), apparent diffusion coefficient (ADC), central nervous system (CNS), cerebrospinal fluid (CSF), endothelin-1 (END1), transforming growth factor-beta (TGF-β), intravenous immunoglobulin (IVIG), internal carotid artery (ICA), anterior cerebral artery (ACA), superior cerebellar artery (SCA)
Copyright © belongs to author(s)
All rights reserved.
Any redistribution or reproduction of part or all of the contents in any form is prohibited other than the following:
- you may print or download to a local hard disk extracts for your personal and non-commercial use only
- you may copy the content to individual third parties for their personal use, but only if you acknowledge the website as the source of the material
You may not, except with our express written permission, distribute or commercially exploit the content. Nor may you transmit it or store it in any other website or other form of electronic retrieval system.
Abstract
Reversible cerebral vasoconstriction syndrome (RCVS) is a clinical entity characterized by hyperacute, transient cerebral artery dilation and narrowing, frequently with thunderclap headache, hemorrhage, infarction, cerebral edema, focal neurological deficits, and seizure. Posterior reversible encephalopathy syndrome (PRES) is a disorder characterized by posterior predominant vasogenic cerebral edema, associated with headache, encephalopathy, visual disturbances, and seizures. These syndromes, both presumed vasculopathies with overlap in clinical, etiological, and radiological features, may be part of a continuum or have shared pathophysiology. Both conditions are rare in children, and concurrent presentation has been documented in only a handful of cases. We present the case of a 10-year-old male with Ehlers-Danlos syndrome and hypoplastic left heart syndrome with recent heart transplant, who presented with seizures and encephalopathy after glucocorticoid administration for treatment of presumed transplant rejection, who was found to have multifocal cortical T2 hyperintensities as well as irregularities of the cerebral vasculature, consistent with concurrent diagnoses of RCVS and PRES. In this case report, we review RCVS, PRES, and their overlapping features in children.
Introduction
Reversible cerebral vasoconstriction syndrome (RCVS) and posterior reversible encephalopathy syndrome (PRES) are rare entities of endothelial dysfunction and impaired cerebrovascular autoregulation, hypothesized to possibly represent a spectrum along shared pathophysiology.1 RCVS is a syndrome of transient, multifocal narrowing of the intracranial vessels and associated thunderclap headache, focal neurological deficits, hemorrhagic and ischemic infarction, and seizures.2 PRES is a syndrome of vasogenic cerebral edema with associated encephalopathy, headache, seizures, and visual disturbances.3 Both conditions are rare in children: PRES has an estimated incidence of 0.04% of hospitalized children4 and 0.4% in those admitted to the intensive care unit,5 while the incidence of RCVS is truly unknown, and information in children is limited to several dozen case reports. There are less than ten reported pediatric cases of comorbid RCVS and PRES. We present the case of a 10-year-old male with Ehlers-Danlos syndrome, recent heart transplant, and administration of glucocorticoids while on systemic immunosuppression, who presented with clinical and radiographic features of both syndromes, and ultimately made full recovery.
Methods
A retrospective chart review was performed with the written assent of the patient and consent of his mother. Our institutional review board does not review single case reports.
Clinical Case
A 10-year-old male with a history of hypoplastic left heart syndrome and orthotopic heart transplant and left convexity subdural hemorrhage had symptomatic seizures immediately following transplant; seizures were well-controlled on a regimen of levetiracetam 50 mg/kg/day. He returned to the hospital three months after transplantation with fatigue and cardiac arrhythmia. Despite reported compliance with tacrolimus and mycophenolate, transplant rejection was suspected. Intravenous methylprednisolone 10 mg/kg/day was administered for 5 days until his cardiac biopsy returned negative for rejection, and subsequently methylprednisolone was discontinued. Tacrolimus was in the desired range at 12.7 ng/mL.
Approximately 72 hours after his last dose of steroids, he began exhibiting thunderclap headache, paroxysms of decreased responsiveness, and right upper extremity tonic-clonic movements. Neurological examination was notable for right-sided face, arm, and leg weakness. A loading dose of 30 mg/kg of levetiracetam was given. Blood glucose was normal, but he was noted to be hypertensive to 138/96 mmHg. Baseline blood pressure at a routine transplant appointment two weeks earlier was 108/67 mmHg.
Computed tomography angiography (CTA) of the head (Figure 1), demonstrated multifocal irregularities of both middle cerebral arteries (MCA) and the left posterior cerebral artery (PCA). Subsequent magnetic resonance imaging (MRI) of the brain and magnetic resonance angiogram (MRA) of the head and neck redemonstrated vascular irregularities, as well as gyriform areas of T2 prolongation in both frontal lobes and the left occipital lobe, as well as striatal and white matter lesions (Figure 2). Diffusion-weighted imaging (DWI) did not demonstrate any areas of low diffusivity signifying acute infarction, and there was no evidence of intracranial hemorrhage. Taken together, the clinical and imaging findings were suspicious for concurrent RCVS and PRES. In the absence of other features concerning for central nervous system (CNS) vasculitis, lumbar puncture and cerebrospinal fluid (CSF) studies were deferred. The patient was started on amlodipine 10 mg daily for empiric treatment of vasoconstriction, and he had cessation of thunderclap headache after two days. Repeat MRI and MRA performed six months following presentation demonstrated resolution of vascular and parenchymal abnormalities.
Discussion
RCVS and PRES are both infrequent neurological disorders, especially in children. RCVS is a clinical and radiological entity characterized by hyperacute onset of reversible, multifocal narrowing of the intracranial arteries, classically associated with thunderclap headache, hemorrhage, infarction, cytotoxic edema, and focal neurological deficits. While frequently idiopathic, RCVS can be provoked by vasoconstrictive drugs, pregnancy, and systemic illnesses.2 PRES is classically associated with parieto-occipital vasogenic edema and headache, encephalopathy, visual disturbances, and seizures. The moniker may be deceptive, in that PRES is not always posteriorly predominant and as many as 20% experience long-term neurological sequelae.3 “Atypical” PRES with a nonclassical distribution of brain abnormalities often involving the basal ganglia, frontal lobes, and brainstem is more common in children; hemorrhage and infarction may be seen in severe cases.6 Uncontrolled hypertension, pre-eclampsia and eclampsia, cytotoxic agents, and renal dysfunction have all been implicated in the development of PRES.3
Shared radiological features of PRES and RCVS, similar inciting etiologies, and overlapping clinical features have led some to hypothesize a common pathophysiology, or even that both syndromes occur along a spectrum.1 (Table 3) Coexistence of PRES occurs in 17-38% of those with RCVS.2,7 Both syndromes have an acute, self-limited, and usually monophasic course involving headaches, encephalopathy, seizures, and focal neurological deficits. Both are associated with pregnancy, especially eclampsia, as well as hypertension, immunosuppressants, autoimmune syndromes, sepsis, and intravenous immunoglobulin.2 There is a paucity of documented cases of pediatric RCVS, and even fewer recognized cases with overlapping features of PRES and RCVS in children (Table 4).8-15 The clinical and imaging features may also overlap with other disorders such as central nervous system vasculitis.
PRES is thought to be a disorder of small-sizes arterioles, while RCVS is characterized by medium- and large-sized arterial dysfunction.8 Some have hypothesized that RCVS progresses centripetally from small, distal vessels to proximal medium- and large-sized arteries. Thus, cerebral angiography is often normal in the early stages in the disease, and helps explain why intracranial hemorrhage is typically seen in the first week and ischemia in the second week as vasoconstriction propagates.2
Chen and Wang16 recently proposed a model of the pathophysiology of RCVS, in which both predisposing and precipitating factors lead to endothelial dysfunction, sympathetic overactivity, and oxidative stress, resulting in disruption of the blood-brain barrier and failure of cerebrovascular autoregulation. Endothelial dysfunction is proposed as the common pathophysiologic mechanism for both RCVS and PRES. Endothelial dysfunction occurs in PRES due to either endogenous or exogenous cytokine release, mitigated by endothelial activation, vascular permeability, and vasogenic edema.3 Vasodilation in response to hypercapnia, an endothelium-dependent process, is impaired in RCVS, and highlights the impaired cerebrovascular tone in this condition.17 Elevated levels of catecholamines may elicit both vasoconstriction and vasodilation within the cerebral arteries of the same individual due to varying distribution of vasoactive receptors. Chen, et al., found that circulating microRNAs related to cerebral vascular tone and endothelial function, such as endothelin-1 (EDN1) and genes involved in the transforming growth factor-beta (TGF-β) could help differentiate those with RCVS from healthy controls.18 No definitive studies elucidating a common pathophysiological mechanism for RCVS and PRES exist, and classically, isolated RCVS occurs in the absence of vasogenic cerebral edema and isolated PRES occurs in the absence of cerebral vasoconstriction. Whether the two conditions are on a continuum within the same syndrome remains speculative.
There are no specific disease-modifying therapies for PRES. Treatment focuses on identifying and removing inciting triggers, and symptomatic management including addressing hypertension and dramatic fluctuations in blood pressure, anti-seizure medications as necessary, and prompt delivery of the fetus if secondary to preeclampsia or eclampsia.3 In RCVS, similar principles apply, including removal of exacerbating conditions, hemodynamic stability, and monitoring for secondary complications. Calcium channel blockers are recommended to promote vasodilation. These have been shown to improve headache severity, yet evidence that they change radiologic or clinical outcomes after RCVS is lacking.2 Transcranial Doppler (TCD) may be useful for monitoring these patients, with several studies suggesting that TCD velocities increase in those with RCVS.19-21 Avoidance of glucocorticoids, triptans, noradrenergic, serotoninergic, and other exacerbating medications is recommended.
Glucocorticoids, as well as tacrolimus and mycophenolate, were administered to our patient immediately prior to symptom onset. PRES has been linked to glucocorticoid administration in both adults and children, especially when coupled with concurrent cancer, active chemotherapy, or active immunosuppression, as in our patient.3 Steroid administration in RCVS is associated with poorer radiologic and clinical outcomes.2 Tacrolimus, even in the absence of PRES, can produce insomnia, tremors, and encephalopathy, and PRES attributed to tacrolimus can occur at therapeutic levels;22 whether tacrolimus toxicity constitutes a separate entity or is also part of this spectrum is another consideration. Avoiding triggering medications is challenging when ongoing immunosuppression is required in patients with organ transplants, and transitioning from systemic immunosuppression can be challenging.
Our admittedly limited data suggest a male predominance for concurrent RCVS and PRES in children – seven of eight cases. A review of available cases of pediatric RCVS found a majority (65.5%) occurred in males,23 in contrast to the adult population, where the majority of RCVS cases occur in females.2 Both Thavamani, et al., and Raj, et al., found that pediatric PRES had a female predominance, of 56.5% and 60%, respectively,4,5 which is congruent with adult data.24
Two of the described cases of dual pediatric PRES and RCVS occurred in individuals with sickle cell disease (SCD) who subsequently received blood transfusion. Individuals with SCD are thought to be at increased risk of PRES,25 in part due to known endothelial dysfunction in this population. Chronic anemia leads to hypoxia, and hemolysis will deplete levels of nitric oxide, both of which contribute to endothelial dysfunction.26,27 In addition, leukocyte activation and platelet dysfunction seen in these individuals leads to increased levels of proinflammatory cytokines, similarly increasing endothelial dysfunction.28 Sudden increases in hemoglobin are hypothesized to decrease physiologic cerebrovascular vasodilation, thereby increasing vascular tone and inducing nitrogen oxide scavenging, both may produce endothelial injury and subsequent vasogenic edema.29,30 While definitive data are lacking, individuals with SCD may be at increased risk of developing PRES and RCVS, presumably during crises when hemolysis and transfusions occur.
A prior case of RCVS complicated by PRES was documented in a boy with Loeys-Dietz syndrome,10 an autosomal dominant connective tissue disorder due to mutation in TGFBR2, which occurred two months after aortic replacement following aortic root dilatation and aortic dissection. Akazawa, et al.,10 hypothesized that aberrant endothelial TGF-β signaling, which has been demonstrated in Loeys-Dietz syndrome,31 may have contributed. Our patient had previously undergone genetic testing for connective tissue disease, due to joint hyperlaxity and congenital heart disease. Testing revealed two heterozygous variants of uncertain significance in LZTS1 (c.1028G>A; p.Arg343Gln), the leucine-zipper, putative tumor suppressor-1, which has been associated with autosomal dominant forms of hypermobile Ehlers-Danlos syndrome, but whether it constitutes a form of vascular Ehlers-Danlos syndrome is unclear.32 Further studies on impairment in cerebral autoregulation and endothelial dysfunction in those with connective tissue disorders are warranted.
Obtaining cerebrovascular imaging for patients with PRES to assess for comorbid RCVS has been suggested but is not widely considered standard of practice. While MRA has been validated for evaluation for RCVS in adults,33 its validation was based on proximal branches of intracranial arteries and may miss more subtle, distal irregularities, such as those seen in our patient. Performing MRA through the whole head to include the distal arteries may increase the yield. Whether cerebrovascular imaging should be performed, and whether MRA or CTA is the more optimal modality, in children with PRES and concern for concurrent RCVS has yet to be fully elucidated.
Conclusion
RCVS and PRES are rare diseases in the pediatric population. This case highlights the clinical and radiologic overlap between RCVS and PRES, and raises the question of whether broader diagnostic consideration, including cerebrovascular imaging, is indicated in children with PRES to assess for comorbid RCVS. The small number of published cases of concurrent pediatric RCVS and PRES limits firm conclusions. Given the paucity of the current literature, further case reports and future research would be beneficial contributions.
References
- Jeanneret V, Jillella DV, Rangaraju S, et al. PRES and RCVS: Two Distinct Entities or a Spectrum of the Same Disease? J Stroke Cerebrovasc Dis. 2022;31(6):106472.
- Singhal AB, Hajj-Ali RA, Topcuoglu MA, et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011;68(8):1005-1012.
- Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14(9):914-925.
- Thavamani A, Umapathi KK, Puliyel M, Super D, Allareddy V, Ghori A. Epidemiology, Comorbidities, and Outcomes of Posterior Reversible Encephalopathy Syndrome in Children in the United States. Pediatr Neurol. 2020;103:21-26.
- Raj S, Overby P, Erdfarb A, Ushay HM. Posterior reversible encephalopathy syndrome: incidence and associated factors in a pediatric critical care population. Pediatr Neurol. 2013;49(5):335-339.
- Saad AF, Chaudhari R, Wintermark M. Imaging of Atypical and Complicated Posterior Reversible Encephalopathy Syndrome. Front Neurol. 2019;10:964.
- Ducros A, Fiedler U, Porcher R, Boukobza M, Stapf C, Bousser M-G. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: frequency, features, and risk factors. Stroke. 2010;41(11):2505-2511.
- Agarwal R, Davis C, Altinok D, Serajee FJ. Posterior reversible encephalopathy and cerebral vasoconstriction in a patient with hemolytic uremic syndrome. Pediatr Neurol. 2014;50(5):518-521.
- Kamide T, Tsutsui T, Misaki K, et al. A Pediatric Case of Reversible Cerebral Vasoconstriction Syndrome With Similar Radiographic Findings to Posterior Reversible Encephalopathy Syndrome. Pediatr Neurol. 2017;71:73-76.
- Akazawa Y, Inaba Y, Hachiya A, et al. Reversible cerebral vasoconstriction syndrome and posterior reversible encephalopathy syndrome in a boy with Loeys-Dietz syndrome. Am J Med Genet A. 2015;167A(10):2435-2439.
- Manuel AR, Gonçalves C, Silva A, Escobar C, Manaças R, Luís C. Reversible Cerebral Vasoconstriction Syndrome in a Previously Healthy Child: A Case Report. Neurol Clin Pract. 2022;12(5):e116-e120.
- Ninomiya H, Kubota K, Kimura T, Kawamoto N, Fukao T. Immunoglobulin A vasculitis complicated with posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome. Pediatr Int. 2019;61(8):836-838.
- Durrleman C, Naggara O, Grevent D, et al. Reversible cerebral vasoconstriction syndrome in paediatric patients with systemic lupus erythematosus: implications for management. Dev Med Child Neurol. 2019;61(6):725-729.
- Regling K, Pomerantz D, Narayanan S, et al. Reversible Cerebral Vasoconstriction Syndrome and Sickle Cell Disease: A Case Report. J Pediatr Hematol Oncol. 2021;43(1):95-98.
- Black M, Cox G, Butler I, Fraser S. Reversible Cerebral Vasoconstriction and Subarachnoid Hemorrhage following Blood Transfusion in a Pediatric Patient with Sickle Cell Disease. Pediatr Stroke. 2023;6:11-20.
- Chen S-P, Wang S-J. Pathophysiology of reversible cerebral vasoconstriction syndrome. J Biomed Sci. 2022;29(1):72.
- Topcuoglu MA, Chan S-T, Silva GS, Smith EE, Kwong KK, Singhal AB. Cerebral vasomotor reactivity in reversible cerebral vasoconstriction syndrome. Cephalalgia. 2017;37(6):541-547.
- Chen S-P, Chang Y-A, Chou C-H, et al. Circulating microRNAs Associated With Reversible Cerebral Vasoconstriction Syndrome. Ann Neurol. 2021;89(3):459-473.
- Hathidara M, Patel NH, Flores A, Cabrera Y, Cabrera F, Koch S. Transcranial Doppler findings in reversible cerebral vasoconstriction syndrome. J Neuroimaging. 2022;32(2):345-351.
- Levin JH, Benavides J, Caddick C, et al. Transcranial Doppler Ultrasonography As a Non-Invasive Tool for Diagnosis and Monitoring of Reversible Cerebral Vasoconstriction Syndrome. R I Med J (2013). 2016;99(9):38-41.
- Oliveira R, Inácio N, Baptista P, Gil-Gouveia R. Transcranial Doppler findings in a population with clinical probable reversible cerebral vasoconstriction syndrome. Rev Neurol (Paris). 2022;178(4):385-390.
- Braithwaite HE, Darley DR, Brett J, Day RO, Carland JE. Identifying the association between tacrolimus exposure and toxicity in heart and lung transplant recipients: A systematic review. Transplant Rev (Orlando). 2021;35(2):100610.
- Maldonado-Soto AR, Fryer RH. Reversible cerebral vasoconstriction syndrome in children: an update. Semin Pediatr Neurol. 2021;40:100936.
- Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427-432.
- Solh Z, Taccone MS, Athale SMU, Breakey VR. Neurological PRESentations in Sickle Cell Patients Are Not Always Stroke: A Review of Posterior Reversible Encephalopathy Syndrome in Sickle Cell Disease. Pediatr Blood Cancer. 2016;63(6):983-989.
- Belcher JD, Mahaseth H, Welch TE, et al. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am J Physiol Heart Circ Physiol. 2005;288(6):H2715-H2725.
- Akinsheye I, Klings ES. Sickle cell anemia and vascular dysfunction: the nitric oxide connection. J Cell Physiol. 2010;224(3):620-625.
- Nader E, Romana M, Connes P. The Red Blood Cell-Inflammation Vicious Circle in Sickle Cell Disease. Front Immunol. 2020;11:454.
- Singh K, Gupta R, Kamal H, Silvestri NJ, Wolfe GI. Posterior reversible encephalopathy syndrome secondary to blood transfusion. J Clin Neurosci. 2015;22(3):592-594.
- Yu B, Lei C, Baron DM, Steinbicker AU, Bloch KD, Zapol WM. Diabetes augments and inhaled nitric oxide prevents the adverse hemodynamic effects of transfusing syngeneic stored blood in mice. Transfusion. 2012;52(7):1410-1422.
- Gallo EM, Loch DC, Habashi JP, et al. Angiotensin II-dependent TGF-β signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J Clin Invest. 2014;124(1):448-460.
- Scicluna K, Formosa MM, Farrugia R, Borg I. Hypermobile Ehlers-Danlos syndrome: A review and a critical appraisal of published genetic research to date. Clin Genet. 2022;101(1):20-31.
- Chen S-P, Fuh J-L, Wang S-J, et al. Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann Neurol. 2010;67(5):648-656.
Figure / Table Legends
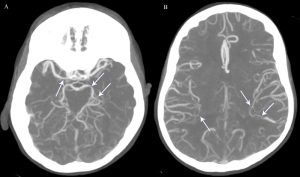
Figure 1:
Computed tomography angiographic maximum intensity projection images obtained on day of presentation, from inferior to superior, demonstrating multifocal luminal narrowing (arrows) of the right proximal MCA and left PCA (A) and distal branches of both MCAs (B). These findings completely reversed on follow-up (not shown).
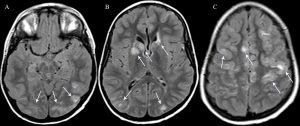
Figure 2:
Multiple T2-weighted FLAIR magnetic resonance images of the brain from inferior to superior show multiple areas of abnormal hyperintensity (arrows) within the occipital lobes (A, B), caudates, basal ganglia, and frontal periventricular white matter (B), and bilateral frontal lobes (C). There was no abnormal low diffusivity or hemorrhage, and the abnormalities resolved on follow-up (not shown). The findings are characteristic of atypical PRES.
See PDF Below for Tables
Reversible cerebral vasoconstriction syndrome complicated by posterior reversible encephalopathy